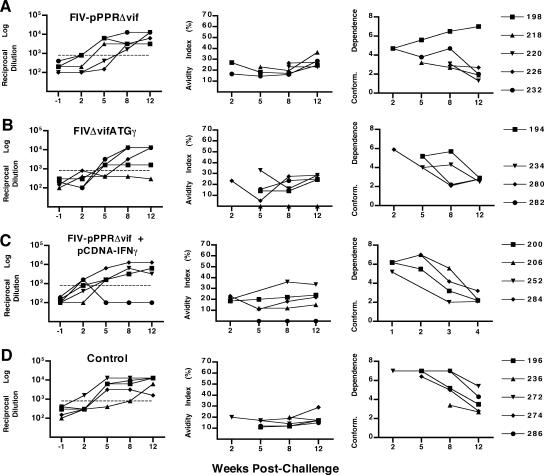
FIG. 5.
Measurement of envelope-specific antibody responses. Envelope-specific serum antibody responses measured by an FIV ConA ELISA are shown for time points 12 weeks postvaccination (or 1 week prior to challenge) and 2, 5, 8, and 12 weeks postchallenge for cats vaccinated with FIV-pPPRΔvif (A), FIVΔvifATGγ (B), or FIV-pPPRΔvif and pCDNA-IFNγ (C) and for unvaccinated control cats (D). The first column of graphs shows reciprocal endpoint FIV Env antibody titers as measured by reactivity to native viral glycoprotein captured from purified FIV-PPR virus in a ConA ELISA. Due to the high background reactivity measured at preimmunization time points for a proportion of the cats, a reciprocal log dilution value greater than 800 (horizontal dashed line) is considered significant. The second column of graphs shows FIV Env antibody avidity indices measured by a modified ConA ELISA for each experimental group, as described in Materials and Methods. Conformational dependence of serum antibodies to FIV Env is shown in the third column of graphs and reflects the ratio of serum reactivity to native viral glycoproteins to that to denatured viral glycoproteins, as measured by the ConA ELISA. Avidity and conformational dependence of FIV Env antibodies were determined only at postchallenge time points and for those serum samples demonstrating an OD at 450 nm of 1.0 or greater at a 1:50 dilution of serum (generally at Env antibody titers of 1:1,000 or greater). As a result, avidity indices and conformation ratio values for serum samples from cats 216 (B) and 296 (C) were not determined. Results shown represent the mean values for two or more assays. Cat identification numbers are to the right.