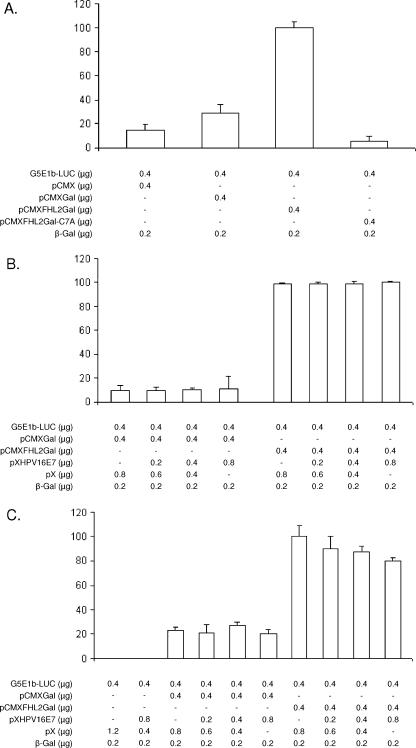
FIG. 2.
E7 fails to influence transactivation by Gal4-FHL2 fusion protein. (A) Transactivation of the Gal4-dependent reporter G5E1b-Luc by Gal-FHL2 fusion protein in NIH 3T3 cells. NIH 3T3 cells were transfected by Lipofectamine with the G5E1b-Luc reporter gene construct (11) in the presence or absence of Gal-FHL2. A cytomegalovirus-driven β-galactosidase (β-Gal) expression vector was cotransfected to control for transfection efficiency. Luciferase activity was normalized to β-Gal activity. The point mutant FHL2Gal-C7A was used as the negative control. Experiments were repeated in triplicate at least three times. Shown are means ± standard deviations (SD) from one representative experiment. (B and C) E7 effects on the activity of Gal-FHL2 fusion proteins. NIH 3T3 cells were transfected with the G5E1b-Luc reporter gene construct in the presence or absence of Gal-FHL2 and increasing amounts of HPV16 E7 expression vector, as indicated. The total amount of DNA was the same (1.8 μg) for each transfection. Cells were serum starved in 0.5% fetal calf serum for 25 h and thereafter either stimulated with 1.2 μM SPP for 3 h (C) or kept in the absence of SPP (B). Luciferase activity was normalized to β-Gal activity. The results are representative of at least three independent experiments. Data shown are the averages of triplicate measurements ± SD.