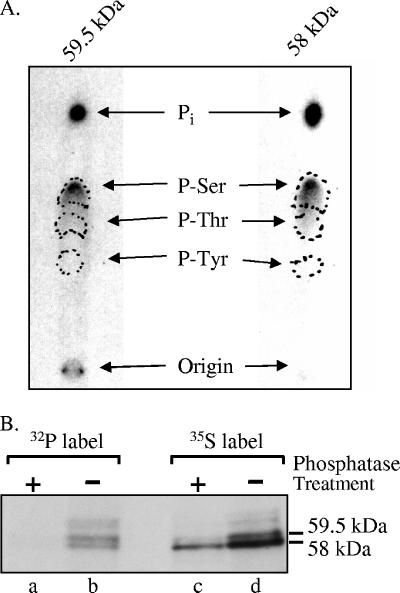
FIG. 2.
Phosphoamino acid analysis of Vhs. (A) The 58-kDa and 59.5-kDa forms of Vhs are phosphorylated predominantly on serines. Vero cells were infected with 20 PFU/cell of wild-type HSV-1 and labeled from 4 to 18 h after infection by incubation in medium containing [32P]orthophosphate. After preparation of whole-cell lysates, the 58-kDa and 59.5-kDa forms of Vhs were immunoprecipitated and purified by preparative SDS-PAGE. Both forms were acid hydrolyzed and mixed with unlabeled phosphoamino acid standards, and the phosphoamino acids were analyzed by 1-D high-voltage electrophoresis on cellulose thin-layer plates. Unlabeled phosphoamino acid standards were localized by staining the plate with ninhydrin, while 32P-labeled amino acids were visualized by autoradiography. The locations of phosphoserine, phosphothreonine, and phosphotyrosine standards are indicated by the dashed circles. (B) The difference in electrophoretic mobility of the 58-kDa and 59-kDa Vhs forms is due primarily to differences in the extent of phosphorylation. Vero cells were infected with 20 PFU/cell of wild-type HSV-1 and labeled from 3.5 to 19 h after infection by incubation in medium containing either [35S]methionine (lanes c and d) or [32P]orthophosphate (lanes a and b). After preparation of whole-cell lysates, the Vhs polypeptides were immunoprecipitated and treated with potato acid phosphatase (lanes a and c) or left untreated (lanes b and d). The products were analyzed by SDS-PAGE and autoradiography. The locations of the 58-kDa and 59.5-kDa forms of the Vhs polypeptide are indicated to the right of lane d.