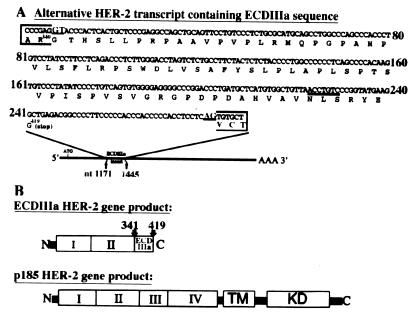
Figure 1.
Nucleotide sequence and deduced amino acid sequence encoded by the 274-nt insertion into HER-2 mRNA. The HER-2 ECD coding sequence from exons 1–9 was amplified by PCR from a cDNA library from SKOV-3 cells. A product of ≈1,420 bp, found to be HER-2 specific by Southern blot analysis, was subcloned, and the nucleotide sequence was determined. (A) The nucleotide sequence is shown for the 274-nt insertion (outside the box) and for the adjacent 5′ and 3′ sequences, enclosed in the box. The insertion is located between nucleotide residues 1171 and 1172 and after amino acid residue 340 in p185HER-2 by using the numbering of Coussens et al. (4). The consensus 5′ and 3′ splice sites are underlined and shown in larger print. The inserted sequence is in-frame with 5′ HER-2 exon sequence and is deduced to encode a 79-aa extension after Arg-340 (R340). A consensus asparagine-linked glycosylation site is underlined. Comparison of the inserted nucleotides and their predicted amino acid sequence with sequences in GenBank showed no obvious homologies. (B) The predicted product of the alternative transcript is a truncated secreted protein that contains subdomains I and II identical to p185 and is missing the transmembrane domain and cytoplasmic domain. If fully glycosylated, the expected size is 65–70 kDa. For comparison, the schematic structure of p185 HER-2 indicates subdomains I, II, III, and IV in the ECD, the transmembrane domain (TM), and the kinase domain (KD).