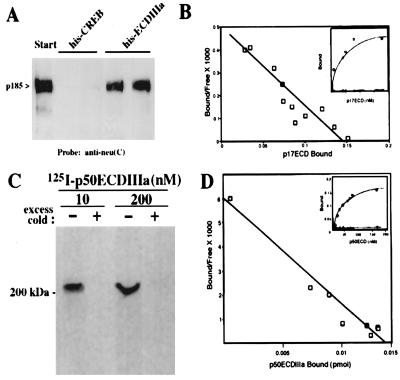
Figure 3.
The ECDIIIa protein specifically associates with p185HER-2. (A) 17-3-1 cell extract (100 μg) wase incubated in duplicate with 50 μl of packed volume of Ni-nitrilotriacetic acid agarose coupled to 20 μg of His-tagged ECDIIIa peptide containing the intron-encoded 79 residues or to 20 μg of His-tagged CREB fragment in 200 μl of wash buffer (20 mM Tris, pH 8.0/300 mM NaCl) at room temperature for 1 hr with shaking. The resin then was washed four times with 500 μl of wash buffer, and proteins were eluted by incubation with 50 μl of SDS-sample buffer at 100°C for 2 min. Eluted proteins were analyzed by Western blot analysis using antibodies against the C terminus of p185, anti-neu(C). (B) Various concentrations of radiolabeled His-tagged ECDIIIa peptide, p17, were incubated with HER-2-transfected 17-3-1 cells or parental 3T3 cells. Binding results were analyzed by using the Scatchard method and by plotting the saturation curve (Inset). (C) Radiolabeled p50 was bound to 17-3-1 cells and then incubated with the crosslinking reagent BS3. The washed cells were extracted and immunoprecipitated with 5 μl of anti-neu(C) as described (26). The immune complex was washed and resolved by SDS/PAGE, and radiolabeled complexes were detected by autoradiography. (D) Various concentrations of radiolabeled p50, purified from bacteria, were incubated with 17-3-1 cells or parental 3T3 cells. Binding results were analyzed by using the Scatchard method and by plotting the saturation curve (Inset).