Abstract
With little improvement in the poor prognosis for humans with high-grade glioma brain tumors, alternative therapeutic strategies are needed. As such, selective replication-competent oncolytic viruses may be useful as a potential treatment modality. Here we test the hypothesis that defects in the interferon (IFN) pathway could be exploited to enhance the selective oncolytic profile of vesicular stomatitis virus (VSV) in glioblastoma cells. Two green fluorescent protein-expressing VSV strains, recombinant VSV and the glioma-adapted recombinant VSV-rp30a, were used to study infection of a variety of human glioblastoma cell lines compared to a panel of control cells, including normal human astrocytes, oligodendrocyte precursor cells, and primary explant cultures from human brain tissue. Infection rate, cell viability, viral replication, and IFN-α/β-related gene expression were compared in the absence and presence of IFN-α or polyriboinosinic polyribocytidylic acid [poly(I:C)], a synthetic inducer of the IFN-α/β pathway. Both VSV strains caused rapid and total infection and death of all tumor cell lines tested. To a lesser degree, normal cells were also subject to VSV infection. In contrast, IFN-α or poly(I:C) completely attenuated the infection of all primary control brain cells, whereas most glioblastoma cell lines treated with IFN-α or poly(I:C) showed little or no sign of protection and were killed by VSV. Together, our results demonstrate that activation of the interferon pathway protects normal human brain cells from VSV infection while maintaining the vulnerability of human glioblastoma cells to viral destruction.
The majority of primary brain tumors display a malignant clinical course, with glioblastoma multiforme being the most frequent and most aggressive form. The prognosis is dismal, with a mean survival of 1 year despite advances in multidisciplinary treatment strategies, including surgery, irradiation, and chemotherapy. New investigative treatment modalities such as replication-competent oncolytic viruses may be particularly effective due to their ability to selectively proliferate within the tumor, leading to a self-amplification of the antitumor effect and intratumoral spread.
In a previous study, we found vesicular stomatitis virus (VSV) to have the strongest selective oncolytic action on human glioblastoma cells out of a panel of nine different viruses that were compared (42). VSV is an enveloped, negative-strand RNA virus belonging to the Rhabdoviridae family. Its 11.2-kb genome consists of five protein-encoding genes (N, P, M, G, and L). VSV causes mild disease in livestock. Infection in humans is rare and is usually asymptomatic, with sporadic cases of mild flu-like symptoms (28). We adapted VSV through multiple passages on glioblastoma cells to generate a modified VSV phenotype, VSV-rp30a (42).
Glioblastoma brain tumors are susceptible to VSV oncolysis (2, 7, 11), though the mechanisms or distinct characteristics that contribute to this effect remain largely elusive. Glioblastomas are extremely heterogeneous in their genotypic and phenotypic makeups. Common aberrations include epidermal growth factor receptor amplification, p53 and PTEN mutations, p16 deletions, and chromosomal aberrations (22). Links of viral oncolytic actions to certain tumor-associated mutations have been established for a variety of potential antitumor agents. Here we sought to address what facilitates selective VSV targeting and oncolysis of glioblastoma cells. Several hypotheses for selective VSV targeting have been suggested. A defective interferon (IFN) system has been postulated (21, 36) for VSV oncolytic action for a variety of peripheral tumors, including colon (36), breast (12), prostate (1), and liver (34), or mesenchymal tumors such as leukemia (18). The innate cellular immune response to VSV infection has not been tested for brain tumors. Another hypothesis argues that there is an affinity of VSV for hypoxic tumor environments (7). A third hypothesis argues that changes in tumor cell translational regulation (3) may be a critical mechanism of VSV oncolysis.
Compared to many other organs, the interferon system appears to be quite distinct in the brain (4). The blood-brain barrier limits the access of circulating IFNs and many IFN-responsive cells to the brain (8), making an effective local IFN system even more important. Systemic IFN may penetrate into the brain at restricted leaky areas, could find access through specific carriers, or could bind to brain endothelial cells and initiate the generation of central modulators (41). IFN-α and -β, also known as type I IFN, are expressed in many cells as an early response to viral infection. In the brain, expression of IFN-α/β could be shown in astrocytes and microglial cells (38, 43). Effects of IFN-α/β on microglial cells, astrocytes, and neurons have been described (6, 19, 30, 39). In contrast, little is known about the antiviral repertoire of glioblastoma cells.
The fatal nature of glioblastoma and the lack of successful treatments suggest alternative approaches are needed. To specifically target tumors in general and glioblastomas in particular, it is important to characterize tumor-specific defects that could be exploited with appropriate means. Here we show interferon deficiencies at different levels that appear to promote effective VSV oncolysis of human glioblastoma cells. Furthermore, we show that boosting the innate antiviral defense with IFN-α and the IFN-α/β pathway inducer polyriboinosinic polyribocytidylic acid [poly(I:C)] provides protection for human astroglial cells, the normal cell type most closely related to glioblastoma cells, but does not block the ability of either VSV or VSV-rp30a to kill glioblastoma cells.
MATERIALS AND METHODS
Glioma cell lines.
The human glioblastoma cell lines U-87MG and MO59J were obtained from the ATCC (Manassas, VA), and cell lines U-373, U-118, and A-172 were kindly provided by R. Matthews and propagated in minimal essential medium supplemented with 10% fetal bovine serum. Medium for U-87 and U-373 cells additionally contained 1% sodium pyruvate and 1% nonessential amino acids.
Normal human control cells.
Normal human astrocytes and oligodendrocyte precursor cells were purchased from Sciencell (San Diego, CA) and kept in culture using astrocyte growth medium or oligodendrocyte medium. In addition, primary astrocyte cultures were established from human brain tissue obtained from epilepsy surgery from three patients different in age and gender and designated A, B, and C. Patient welfare was the sole basis for resection of brain tissue. Tissue blocks were cut into small cubes and plated onto Millipore filter insets or poly-l-lysine coated coverslips for explant cultures. Cells were harvested and propagated in minimal essential medium with 10% fetal bovine serum.
Red fluorescent protein (RFP)-expressing U-87 cells.
To generate human glioblastoma cells that expressed a colored reporter gene, U-87 cells were plated in 25-cm2 tissue culture flasks. Confluent cultures were transfected with plasmid pDsRed-Monomer-C1 (Clontech), containing an expression cassette with dsRed driven by a cytomegalovirus promoter and a neomycin resistance gene, by using Lipofectamin2000 (Invitrogen). G418 (Sigma) was used to select and maintain stably transfected U-87 cells.
All cells were propagated in a humidified atmosphere containing 5% CO2 at 37°C.
Viruses.
Green fluorescent protein (GFP)-expressing VSV (9) and VSV-rp30a, derived from VSV-rp30 (42), were generated as previously described. Titers for VSV and VSV-rp30a were determined through plaque assay on BHK cells.
Quantitative real-time PCR.
Glioblastoma cells or control cells were grown in 25-cm2 flasks in triplicates for each treatment condition. Ninety percent confluent cultures were treated with either IFN-α (100 u/ml), poly(I:C) (25 μg/ml), or VSV-rp30a (multiplicity of infection [MOI] of 2), or mock treated, for 6 h. Cells were lysed, and RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA) was used to reverse transcribe 1 μg of total RNA, using random hexamers. Quantitative PCR was performed on duplicate 25-μl PCR mixtures containing 100 ng reverse-transcribed RNA, 12.5 μl SYBR green reaction mix (Applied Biosystems), and 200 nM sense and antisense primers. Unless otherwise specified, all tests were run on at least three independent cell samples per group. Reactions were run using an Applied Biosystems 7500 real-time PCR system. After an initial incubation at 95°C for 5 min, PCR amplification was performed by cycling 50 times for 1 min at 95°C followed by 1 min at 60°C. Gene expression was quantified using the 7500 system sequence detection software (Applied Biosystems) after normalization to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression.
Cell growth and viability.
Cells were plated at a density of 5,000 per well in 96-well dishes and incubated overnight before medium (0.1 ml per well) was replaced and virus was added. Viability was assessed using an MTT (Molecular Probes) assay according to the manufacturer's instructions. Optical density was read at 570 nm using a Dynatech MR500 enzyme-linked immunosorbent assay plate reader (Dynatech Lab Inc., Alexandria, VA) and corrected from a background control. Each condition was tested in triplicate.
Cytotoxicity assay.
Cells were seeded in 24-well plates at a density of 50,000 cells per dish. After 12 h, 1 ml serum-free medium containing 5 × 105 PFU (MOI, 5) of VSV, VSV-rp30, or no virus (control) was added, and 6 h later, fetal bovine serum was added to each dish to make a final concentration of 10%. GFP expression was recorded at the indicated time points. Ethidium homodimer (EthD-1; Molecular Probes, Eugene, OR) was used to label dead cells; 20 μl of EthD-1 stock was dissolved in 10 ml PBS (with Ca2+ and Mg2+). Medium was carefully removed and replaced by 250 μl EthD-1 solution per dish. After 40 min of incubation at 37°C, dead cells were detected based on red fluorescence of nuclei.
Coculture time-lapse recordings.
Normal human astrocytes or explant cultures from normal human brain tissue were plated onto 35-mm culture dishes with glass bottoms (MatTek Co., Ashland, MA) at medium density. After 1 day, 50,000 to 100,000 U-87 cells expressing dsRed were added to the dish to form cocultures. Pretreatment was started 12 h later and involved a medium change to Leibovitz's L-15 medium (Gibco) containing 10% fetal bovine serum and pretreatment drugs consisting of human IFN-α (Sigma-Aldrich; catalog no. I4401) or poly(I:C) (Amersham) at the indicated concentrations. At 6 to 12 h later, virus was added to reach an MOI of 5. The dish was sealed and placed on a heated (37°C) stage of an Olympus IX-81 fluorescence microscope. The focus, field coordinates, fluorescence shutter, filter revolver, and Hamamatsu digital camera were all computer controlled using Slidebook software (Intelligent Imaging Innovations Inc., Denver, CO). Images were recorded for GFP (virus), dsRed (human glioblastoma cells), and phase contrast (normal, non-red human astrocytes) at 6-min intervals. The total recording periods varied between 50 and 80 h.
RESULTS
We and others have previously shown that cell lines derived from human glioblastomas are highly susceptible to VSV-mediated oncolysis (2, 7, 11, 42). To determine whether this is due to specific deficits in antiviral defense exhibited by the tumor cells, we compared a panel of five different glioblastoma cell lines with a number of control brain-derived cells for VSV-induced cytolysis in the presence or absence of interferon pathway activation. Consistent with previous findings (42), all glioblastoma cell lines were killed by VSV and the glioma-adapted strain VSV-rp30a (data not shown). VSV-rp30a was substantially faster in its infection rate and oncolytic effects than VSV, but this was seen primarily at early time intervals. At 6 h postinfection, the relative ratios of infected cells (denoted by expression of the GFP reporter) to uninfected cells (mean of 10 random microscope fields) for VSV-rp30a and VSV were quantified. The respective ratios for each type of glioblastoma were 94.1% ± 1.2% and 56.5% ± 6.1% for U-87, 87.8% ± 1.3% and 44.1% ± 4.5% for U-118, 65.4% ± 4.2% and 12.7% ± 1.8% for U-373, and 68.6% ± 2.9% and 8.8% ± 1.1% for A-172. Thus, in all cases, VSV-rp30a infected glioblastoma cells more robustly at this time point than the other VSV strain.
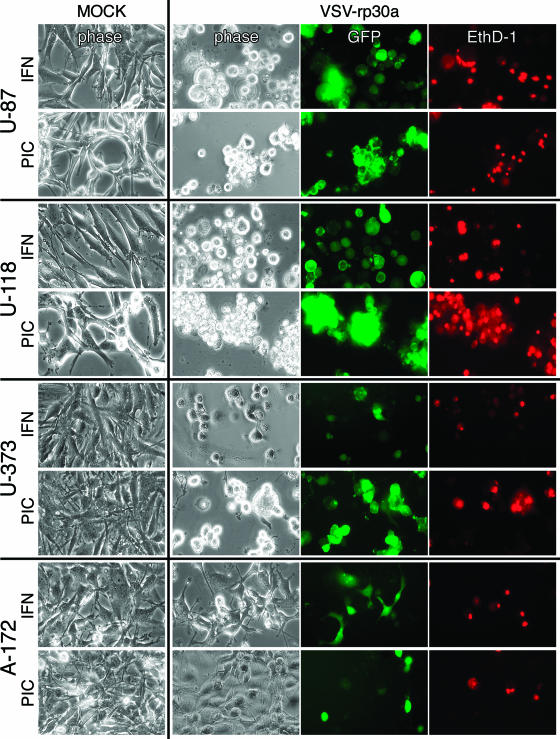
In the absence of IFN-α, all tumor cells were dead or showed pronounced cytopathic effects within 48 h. Preincubation with IFN-α (100 U/ml) or the double-stranded RNA poly(I:C) (25 μg/ml) had only a slight protective effect on tumor cells against VSV and VSV-rp30a at an early time point. Figure 1 displays representative images of four glioblastoma cell types pretreated and infected with VSV-rp30a. U-87 and U-118 were completely infected within 24 h, with most of the cells dead or dying, as indicated by the red fluorescence of the EthD-1 nuclear staining. The course of VSV-rp30a infection of U-373 cells was unaffected by poly(I:C) pretreatment but was moderately attenuated in IFN-α-pretreated cultures, leading to delayed onset of GFP expression and cell death. A-172 glioblastoma cells showed signs of protection from VSV-rp30a infection after IFN-α and poly(I:C) treatment, with fewer than half of the cells expressing GFP or displaying cytopathic effects. Together, these results indicate that three out of four glioblastoma cell lines tested remain highly susceptible to VSV and VSV-rp30a infection even in the presence of IFN-α or after poly(I:C) treatment (see also Fig. 8).
FIG. 1.
VSV action on four glioblastoma cell lines. Four different glioblastoma cultures were infected with VSV-rp30a at an MOI of 5 in the presence of IFN-α (100 IU/ml) or poly(I:C) (PIC) (20 μg/ml). Expression of GFP was used to monitor viral infection; EthD-1 was used to assess virus induced cell death. Representative photomicrographs were taken at 24 h postinfection (U-87 and U-118) or at 48 h postinfection (U-373 and A-172). The presence of IFN or PIC had only a modest impact on the oncolytic action of VSV-rp30a.
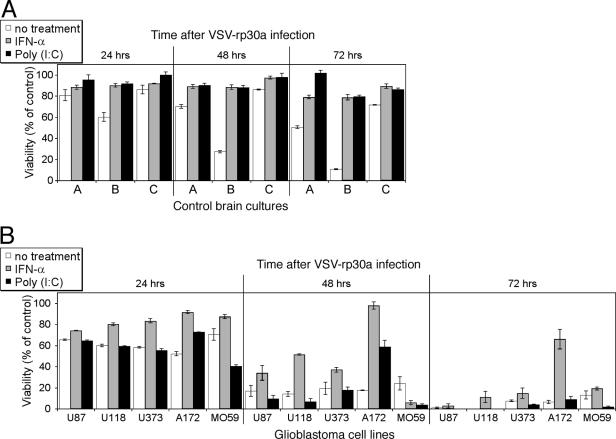
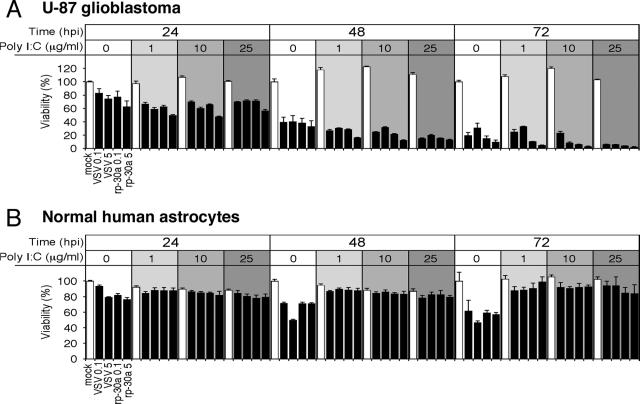
FIG. 8.
Complete protection of normal human brain cultures from VSV-rp30 infection by IFN-α and poly(I:C). The same set of cells used for the gene expression studies (see Fig. 7) was analyzed for cell viability after VSV-rp30a infection (MOI of 5) in the presence or absence of IFN-α and poly(I:C) over a 3-day time course. (A) IFN-α and poly(I:C) offer complete protection for all three control brain cultures. Even without pretreatment, viability was significantly higher after VSV-rp30a infection than in glioblastoma cells. (B) In contrast, all five glioblastoma cell lines were nearly completely killed by VSV-rp30a, and only one cell line showed a moderate response to protective IFN-α pretreatment. There was no protection with poly(I:C). Data represent means of triplicates and standard errors of the means.
IFN-α and poly(I:C) protect normal human astrocytes.
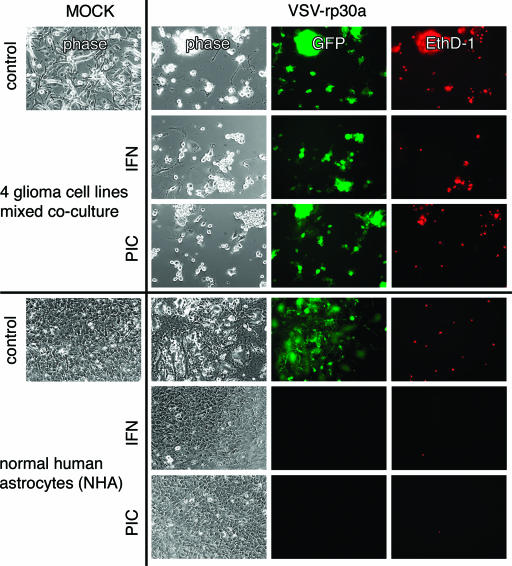
We next compared the effect of IFN-α and poly(I:C) pretreatment on VSV and VSV-rp30a infection of glioblastoma cells with that on infection of normal human astrocyte control cells. GFP expression and cell death were recorded using fluorescence light microscopy for detection of GFP reporter gene expression and EthD-1 cell death labeling. Cells from four different glioblastoma cell lines (see above) were mixed together (Fig. 2). As shown in Fig. 1, incubation with IFN-α or poly(I:C) had little protective effect. Most cells in the mixed tumor culture expressed GFP and displayed significant cytopathic effects in control, IFN-α, and poly(I:C) dishes. Similarly, widespread nuclear staining in the EthD-1 cell death assay indicated substantial tumor cell killing. In contrast, normal human astrocytes were protected from VSV-rp30a infection when pretreated with IFN-α or poly(I:C) (Fig. 2). This protective effect for VSV-rp30a was not different from that for VSV (data not shown). Triplicates of culture dishes were pretreated and infected at an MOI of 5. Even after a 1-week surveillance period, no signs of GFP expression or abnormal morphological changes were observed. Without pretreatment, astrocyte cultures showed signs of VSV infection and infection-related cell death, as indicated by EthD-1 cell death labeling. However, the extents of GFP expression and cell death were lower than those in glioblastoma cell cultures even in the absence of IFN-α prophylactic pretreatment.
FIG. 2.
IFN and poly(I:C) protect normal glial cells, but not glioblastoma cells, from VSV infection. A mixed culture of four glioblastoma cell lines (see Fig. 1) and normal human astrocytes were infected with VSV-rp30a at an MOI of 5. Overnight pretreatment with IFN-α (100 IU/ml) or poly(I:C) (PIC) (20 μg/ml) did not protect tumor cells but did protect control cells from VSV infection and lysis. Viral infection was monitored by expression of GFP, and cell death was assessed by an ethidium homodimer assay. Panel displays representative images taken 24 h (tumor cultures) and 48 h (control cultures) after viral inoculation.
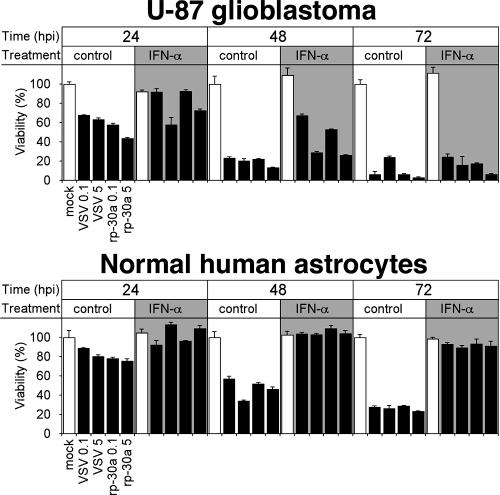
To quantitatively measure the effect of IFN-α on VSV-induced cytopathology of normal human astrocytes and U-87 glioblastoma cells, we performed experiments parallel to those described above but using the MTT cell viability assay. U-87 and normal human astrocytes were grown in 96-well dishes and treated with IFN-α (100 U/ml) overnight prior to addition of the virus. In the course of 3-day infections, IFN-α had little protective effect on the viability of U-87 glioblastoma cells (Fig. 3). Under untreated conditions, both VSV and VSV-rp30a effectively killed nearly all U-87 cells. The action of VSV-rp30a was slightly stronger than that of VSV (P < 0.05 at an MOI of 5; P < 0.3 at an MOI of 0.1). Pretreatment with IFN-α was ineffective at protecting glioblastoma cells (P < 0.001 by analysis of variance). On the other hand, pretreatment with IFN-α completely protected normal human astrocytes from VSV cytotoxicity (Fig. 3). Even in the absence of IFN-α, the effect of VSV infection on viability of astrocytes was significantly less pronounced than in U-87 cells (P < 0.01 by paired t test). Interestingly, viral load appeared to have little effect. The unaffected viability of human astrocytes in the presence of IFN-α is consistent with the lack of GFP expression upon VSV and VSV-rp30a infection mentioned above.
FIG. 3.
Effect of IFN on VSV infection. Using an MTT cell viability assay, the effect of IFN pretreatment on the action of VSV on U-87 cells and normal human astrocytes was assessed. IFN had little effect on glioblastoma cell destruction by VSV but completely protected normal astrocytes. Low (MOI, 0.1) and high (MOI, 5) concentrations of VSV and VSV-rp30a were used. Note the slightly stronger oncolysis by VSV-rp30a. Error bars indicate standard errors of the means from triplicate experiments. hpi, hours postinfection.
Synthetic double-stranded RNA polymers are potent inducers of the endogenous IFN-α/β pathway. If preincubation with poly(I:C) leads to endogenous IFN production in human astrocytes, then a protection from VSV-induced cytopathology would be expected. U-87 glioblastoma cells and normal human astrocytes were treated with poly(I:C) at three different concentrations (1, 10, and 25 μg/ml) for 24 h prior to addition of VSV and VSV-rp30a at MOIs of 0.1 and 5. Preincubation with Poly(I:C) had no protective effect on viability of U-87 cells; in fact, poly(I:C) reduced the viability of glioblastoma cells independent of viral inoculation (P < 0.05) and enhanced the antitumor potency of VSV and VSV-rp30a in a dose-dependent manner (Fig. 4A). In these experiments, VSV-rp30a displayed a moderately stronger antitumor effect than VSV-GFP. In contrast, poly(I:C) preincubation at all concentrations effectively protected normal human astrocytes (Fig. 4B). The cell viability of normal human astrocytes was not impaired by VSV and VSV-rp30a infection even at the low poly(I:C) concentration. Poly(I:C) alone had no effect on cell viability (Fig. 4).
FIG. 4.
Effect of poly(I:C) on VSV infection. Using an MTT cell viability assay, the effect of poly(I:C) pretreatment in several concentrations was assessed. U-87 glioblastoma cells were effectively killed by virus in the absence or presence of poly(I:C). Note the slightly stronger oncolysis by VSV-rp30a. Noteworthy is that in the presence of poly(I:C), VSV action on U-87 cells was even stronger than in nonpretreated cultures, suggesting a synergistic effect of poly(I:C) with VSV/VSV-rp30a. In contrast, normal human astrocytes were completely protected from VSV infection. hpi, hours postinfection.
Together, these data indicate that normal human astrocytes are protected from VSV infection by administration of both exogenous IFN-α and the IFN-α/β pathway inducer poly(I:C).
Effects of IFN and poly(I:C) on viral replication.
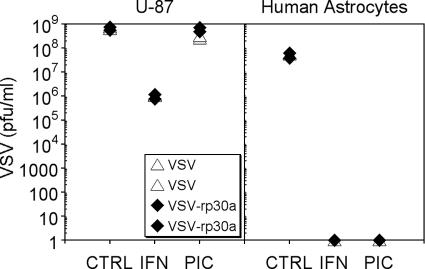
Viral replication was assessed by plaque assay of serial dilutions of supernatants 24 h after infection of U-87 cultures or normal human astrocyte cultures with VSV or VSV-rp30a at an MOI of 1. Experiments were performed in duplicate (Fig. 5). Under control conditions, supernatants of U-87 glioblastoma cultures infected with VSV and VSV-rp30a contained nearly 109 PFU/ml (7.5 × 108 and 6.1 × 108 PFU/ml for VSV and 7.7 × 108 and 5.6 × 108 for VSV-rp30a [values for duplicates of experiments]). Though pretreatment with IFN-α resulted in fewer progeny, indicating moderately attenuated viral replication, glioblastoma cell supernatants still contained about 106 PFU/ml of both VSV and VSV-rp30a. Pretreatment with poly(I:C), on the other hand, had nearly no effect on viral replication in U-87 cells, resulting in PFU concentrations similar to those under nontreated control conditions (3.2 and 2.6 × 108 PFU/ml for VSV and 7.1 and 5.0 × 108 PFU/ml for VSV-rp30a). In striking contrast, there was virtually no viral replication in IFN-α- and poly(I:C)-treated normal human astrocyte control cells, as indicated by the absence of any detectable plaques. Even under nontreated control conditions, viral replication in normal human astrocytes was lower than that in nontreated U-87 cells (6.0 and 5.5 × 107 PFU/ml for VSV and 6.3 and 3.9 × 107 PFU/ml for VSV-rp30a). Together these data indicate that IFN-α and poly(I:C) dramatically reduce viral proliferation in normal cells but not in glioblastoma cells.
FIG. 5.
Effect of IFN-α and poly(I:C) on viral replication. Viral replication was assessed using plaque assays. U-87 glioblastoma cultures and normal human astrocytes were infected at an MOI of 1, washed, and incubated. Supernatant was collected 24 h later. In IFN-α- and poly(I:C) (PIC)-pretreated astrocyte cultures, no viral progeny could be detected. In contrast, viral replication in U-87 cells was mildly attenuated by IFN and unaffected by poly(I:C).
Protective effect of IFN-α on other human central nervous system (CNS) cells.
In addition to normal human astrocytes, we tested whether IFN-α could protect primary human brain tissue derived from epilepsy surgery from VSV infection. Primary explant cultures were established from brain specimens from three patients of different ages and genders, resulting in semiconfluent cell layers with glial morphology. We also tested commercially available human oligodendrocytes (Sciencell, San Diego, CA). In the absence of IFN treatment, both human oligodendrocytes and, to a lesser extent, primary human brain tissue explant cultures showed some GFP expression and cytopathic morphology changes upon VSV infection. In contrast, IFN-α pretreatment (100 U/ml overnight) resulted in complete protection of the respective cells from both VSV and VSV-rp30a infection, and neither cytopathic effects nor GFP expression was detected for up to 6 days postinfection, when the experiment was stopped. Representative photomicrographs from these experiments are displayed in Fig. 6.
FIG. 6.
IFN protection of human brain tissue explant cultures and human oligodendrocyte precursor cells. Primary explant cultures from postsurgery human brain tissue (upper set) and human oligodendrocyte precursor cells (lower set) were infected with VSV-rp30a in the presence or absence of IFN-α. Normal morphology was seen in noninfected control cultures. The primary brain culture used here corresponds to control sample C in Fig. 7 and 8.
Different expressional responses to VSV-rp30a, IFN-α, or poly(I:C).
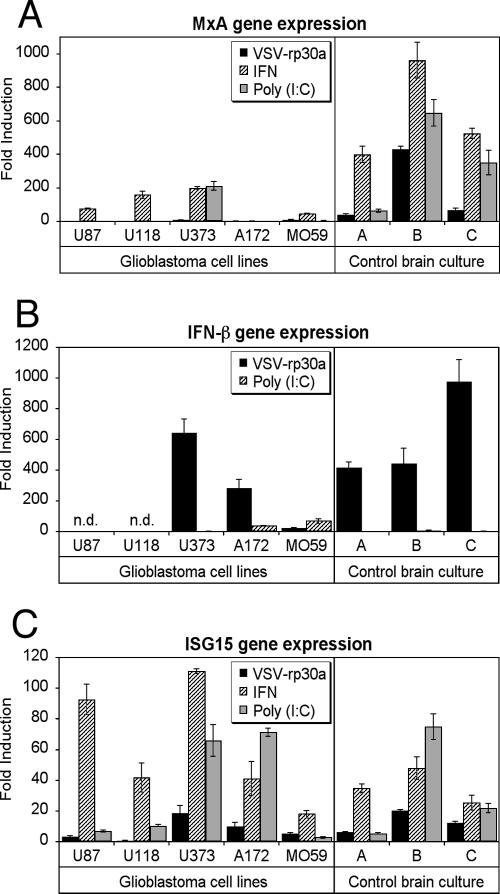
To address further underlying mechanisms that may mediate VSV oncolysis of glioblastoma cells, we used quantitative reverse transcription-PCR (RT-PCR) to examine the expression of IFN-β and two representative well-characterized IFN-α/β-stimulated genes, MxA and ISG15. All five glioblastoma cell lines tested were exquisitely susceptible to oncolytic action of VSV. However, pretreatment with IFN-α or poly(I:C) resulted in various outcomes of VSV infection, suggesting distinct aberrations of antiviral defense mechanisms in different glioblastoma cell lines. Nearly confluent cultures were treated for 6 h with either VSV-rp30a (MOI of 2), IFN-α (100 U/ml), or poly(I:C) (25 μg/ml) or were mock treated. Quantitative RT-PCR analysis revealed striking differences in the expression patterns of IFN-β and downstream IFN-α/β-induced genes between glioblastoma and control cells (derived from three different patients' postsurgical brain specimens). Interestingly, differences were also found between glioblastoma cells of different derivations (Fig. 7). Among the genes examined, the most consistent difference was seen in the expression of MxA, a cellular protein with well-established antiviral activity (25). Upon VSV-rp30a infection, all control cells responded with a significant increase of MxA expression up to 400-fold (40-, 67-, and 430-fold for the three brain derivations) compared to nontreated cultures (Fig. 7A). In contrast, no detectable induction was observed in three tumor cell lines (U-87, U-118, and A-172), and very low responses (<10-fold increase) were observed in the other two. In addition, MxA expression upon IFN-α pretreatment was significantly higher in control cells (up to nearly 1,000-fold compared to nontreated cultures) than in glioblastoma cells (P < 0.01), with three out of four cell lines showing weak to moderate responses and the fifth, A-172, showing no response to IFN. After poly(I:C) treatment, only one out of five glioblastoma cell lines (U-373) responded with a significant induction of MxA expression, compared to a consistent induction in all control cultures.
FIG. 7.
Differences in interferon-related gene expression upon VSV infection in glioblastoma and control brain cells. Cultures of five different human glioblastoma cell lines and three control cultures derived from postsurgery human brain tissue were either infected with VSV-rp30a (MOI of 2) or treated with human IFN-α (100 IU/ml) or poly(I:C) (25 μg/ml) for 6 h. Gene expression studies using quantitative RT-PCR revealed marked differences between glioblastoma and control cells for MxA (A) and IFN-β (B) but not for ISG15 (C). Data are expressed as fold induction relative to control untreated cultures. The level of expression was different for each tested gene (y axes have different scales). Expression levels are normalized to the cellular GAPDH gene and are presented as means of triplicates with standard errors of the means. n.d., none detected.
Next we addressed the question of to what extent VSV infection or poly(I:C) treatment induces IFN-β expression in control and tumor cells. Interestingly, IFN-β expression was highly induced in human primary brain culture cells (400- to 1,000-fold) upon VSV-rp30a infection but not upon poly(I:C) treatment. This is particularly remarkable, as poly(I:C) treatment not only upregulated the IFN-induced gene MxA but also provided complete protection from infection, suggesting that poly(I:C) directly activates expression of IRF3-regulated genes with antiviral activity even in the absence of IFN-β expression, as previously suggested (10). In contrast IFN-β expression was completely absent in U-87 and U-118 glioblastoma cells but was induced in U-373 and A-172 cells upon VSV-rp30a infection. Interestingly, IFN-β expression in A-172 cells did not correspond to any detectable MxA expression, suggesting potential defects downstream of IFN-β expression in these cells.
A second IFN-α/β-stimulated gene, ISG15, is one of the earliest IFN-α/β-induced genes and may play a role in cellular defenses against viruses such as human immunodeficiency virus and Sindbis virus (16), but it does not appear to play a substantial role in protection against VSV (24). We included ISG15 studies to test the hypothesis that although it is an IFN-α/β-induced gene, it would not correlate with IFN-conferred antiviral (VSV) protection. Indeed, ISG15 gene expression levels did not show a strong correlation with antiviral activity, as all glioblastoma cells showed increased ISG15 but were not protected from VSV-mediated oncolysis. ISG15 was only modestly upregulated compared to MxA after VSV-rp30a infection, with 8-fold increases in normal control cells compared to 12-fold increases in four tumor cell lines and a complete absence of response in U-118 cells (Fig. 7C). In addition, all cells (control and glioblastoma) showed ISG15 gene induction after IFN-α or poly(I:C) pretreatment.
Together, these data indicate a heterogeneous pattern of induction of IFN-β and IFN-induced genes by VSV-rp30a infection or IFN-α or poly(I:C) treatment in control cells compared to glioblastoma cells, which may in part explain the susceptibility of glioblastoma cells to VSV oncolysis. In addition, different glioblastoma cells appear to have distinct aberrations in IFN-β induction or downstream antiviral gene expression, which may explain why some glioblastoma cell lines are more effectively killed by VSV than others even in the presence of IFN-α or poly(I:C).
Protection of normal human brain cells from VSV infection.
The gene expression study shown in Fig. 7 was coupled to a functional assessment of antiviral effects of IFN-α and poly(I:C) pretreatment on the same glioblastoma cell lines and normal human control brain cells by using the MTT cell viability assay (Fig. 8). Normal human brain cultures were completely protected by either IFN-α or poly(I:C) pretreatment. Even without treatment, two normal brain cultures showed only moderate decreases in cell viability compared to tumor cells after infection. One normal culture showed impairment of viability after 3 days. Although not the focus of this study, this heterogeneity may be attributed to age, gender, and anatomical origin and cellular composition of the brain specimen. In contrast, only one out of five tumor cell lines (A-172) was incompletely protected by IFN-α pretreatment. Poly(I:C) treatment did not provide any protection for glioblastoma cells. Of note, the cell viability of two glioblastoma cell lines, A-172 and MO59J, was significantly reduced by poly(I:C) treatment even in the absence of viral infection (data not shown), suggesting that these two cell lines were directly sensitive to antitumor actions of poly(I:C).
Fluorescence time-lapse recording of infected astrocyte-glioblastoma cell cocultures.
Glioblastoma cells are highly invasive and often spread deep into the normal brain parenchyma. This distribution of malignant cells widely dispersed in normal tissue presents a major challenge for selective tumor cell killing. Furthermore, unlike most inert reagents, which decrease with time after application, the VSV concentration increases. This raises a question of whether in a mixed normal astrocyte/glioblastoma cell culture, the normal astrocytes would be able to still resist VSV infection when surrounded by tumor cells releasing replication-competent virus.
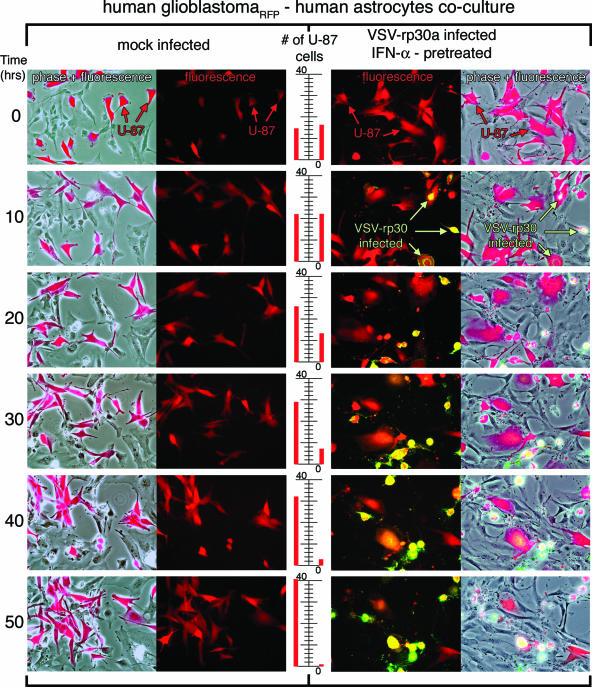
To address this condition in an in vitro experimental model, a coculture experiment was used with red fluorescent U-87 tumor cells seeded into dishes containing subconfluent nonfluorescent normal human astrocytes or primary brain tissue explant cultures. U-87 cells stably expressed RFP (a monomeric form of dsRed); cultures of these cells contained >99% red cells. In control experiments, either RFP glioblastoma cells or normal astrocytes were imaged for 4 days. In the absence of virus, both cell types continued to grow and replicate, indicating that the conditions of digital imaging continuously for an extended period of 4 days were not debilitating to either cell type. Similar controls with IFN-α or poly(I:C) similarly showed strong viability. Cocultures of the two cell types (normal astrocytes and red glioblastoma cells) were pretreated with human IFN-α (200 U/ml) overnight or with poly(I:C) (20 μg/ml) for 6 h. VSV or VSV-rp30a was added at an MOI of 5. Under these conditions, time-lapse recording of infected cocultures confirmed the selective infection and cell lysis of glioblastoma cells but not control cells. Experiments were repeated at least three times for each condition and each virus. Figure 9 displays two sets of representative time-lapse recordings for nontreated, mock-infected control cultures and IFN-α-pretreated, VSV-rp30a-infected cultures. As expected, the number of red tumor cells increased steadily over time in mock-infected cultures (Fig. 9). In this representative series, the number of red glioblastoma cells rose from 14 to 41 cells in the control condition without virus. In contrast, VSV-rp30a selectively infected U-87-dsRed, which expressed viral GFP starting at approximately 6 h postinfection and showed rapid formation of cytopathic effects (rounding up and blebbing). At the end of the recording period, nearly all red tumor cells had been infected and were either dead or showed severe cytopathic effects (the cell number decreased from 16 to 1 in the representative series in Fig. 9). In contrast, normal control cells in the same culture dish were mostly unaffected and showed normal morphology throughout the observation period and did not express GFP; <2% of the control cells showed any signs of infection. A few normal control cells in the immediate vicinity of dying glioblastoma cells showed some morphological changes in the absence of GFP expression. A bystander effect due to the release of toxic molecules by the dying cell may be a possible explanation for this observation.
FIG. 9.
Time-lapse recording of VSV-rp30a selectively infecting and killing glioblastoma cells in coculture with normal human astrocytes. Glioblastoma U-87 cells were stably transfected to express RFP and cocultured with normal human astrocytes. Cultures were pretreated with IFN-α (100 IU/ml) overnight. VSV-rp30a was added at an MOI of 5. The panel displays two representative experiments, one in control conditions without VSV (left) and one with addition of GFP-expressing VSV-rp30a (right). The center scale illustrates the increase of red glioblastoma cell number in control conditions and decrease in the virus-infected culture. Pictures for time-lapse recording were automatically taken every 6 min over a period of 50 h. For the displayed panels, every 100th frame was used, corresponding to 10-hour intervals.
DISCUSSION
As a key element of the current study, we showed that a variety of normal human brain cells are protected from VSV infection by activation of the IFN-α/β pathway. Both direct IFN-α treatment and IFN-α/β induction by poly(I:C) led to complete protection from VSV infection. Human glioblastoma cells, on the other hand, were successfully targeted and destroyed by the virus even after IFN-α pretreatment. The protection of control cells was correlated with a strong induction of IFN-β and MxA gene expression.
We tested a number of different glioblastoma types to reflect the heterogeneous nature of these malignancies. Four out of the five glioblastoma cell lines tested were infected and killed by VSV and VSV-rp30a in the presence of IFN-α or poly(I:C). One cell line (A-172), however, did show significant signs of protection from VSV infection under IFN pretreatment conditions. This rate of IFN insensitivity is consistent with reports on other types of tumor cells, where only a minority were protected by IFN-α treatment (36). Though this indicates that a functional IFN defect is not an absolute hallmark of all tumors, it is nonetheless a feature that may be shared more frequently than many other genetic aberrations.
In contrast to glioblastoma cells, all nonmalignant control brain cells, including astrocytes, oligodendrocytes, and heterogeneous primary human brain cultures, were completely protected by both IFN-α incubation and poly(I:C) pretreatment. The latter effect is important to note, as it underlines the capability of normal human glial cells to respond to poly(I:C) sufficiently to provide full protection from VSV infection. The action of poly(I:C) appears to be facilitated mainly through Toll-like receptor class 3 (TLR3), which has been previously shown to be expressed in the brain (13). TLR3 recognizes double-stranded RNAs (32) generated by many viruses. TLR3 expression on astrocytes may therefore underline the important role of these cells in detecting a virus in the brain. In our study, poly(I:C) was highly effective for antiviral protection of normal human brain cells. However, this effect was not directly coupled to an induction of endogenous IFN-β expression, consistent with direct activation of downstream IFN-α/β-inducible genes. Interestingly, poly(I:C) appears to have some moderately synergistic effect with the oncolytic action of VSV and VSV-rp30a, as indicated by a concentration-dependent decreased viability for U-87 cells (Fig. 4). In addition, poly(I:C) alone had a clear suppressive effect on A-172 and MO59J tumor cell viability. Poly(I:C) has been shown to be effective and safe when given intranasally or intracerebrally (14). In addition to its use in a number of experimental or clinical trials for various viral diseases (20, 37), poly(I:C) has also been tested in a clinical trial for direct treatment of glioblastoma (29). Both IFN and poly(I:C) protected normal brain cells from recombinant VSVs. It is important to note that cells within the normal brain would benefit from many additional antiviral defenses of the systemic immune system, including activated B and T cells and macrophages/microglia, which were minimal or absent in our culture conditions.
Conditional viral replication is a central feature of oncolytic viral use in comparison to vector-based gene therapy strategies that utilize replication-deficient virions. Replication of VSV and VSV-rp30a was only moderately reduced by IFN-α and was not affected by poly(I:C) in U-87 cells; in contrast, little viral replication was detected in nontumor control CNS cells pretreated with IFN-α or poly(I:C). This is particularly important, as it would theoretically prevent further viral spread beyond the interface of tumor mass and normal brain parenchyma. For our replication studies, we used normal human astrocytes, the cell type most closely related to glioblastoma tumors, as control cells. Of note, a similar inhibitory effect of IFN on VSV replication was shown with mouse neuron cultures in a recent report (39). Previous work (42) showed that VSV-rp30 was more effective in killing glioblastoma cells than VSV-GFP; this was particularly noticeable at shorter time intervals. For this reason we used VSV-rp30a for the experiments here. In some experiments we also used VSV-GFP, and we found that VSV-GFP and VSV-rp30 showed similar actions and responses at the longer time intervals in which both viruses had sufficient time to replicate during multiple cycles, producing a final high MOI. A faster-acting virus such as VSV-rp30a may provide some advantage in brain tumors, in which hundreds of millions of tumor cells would ideally be destroyed before a substantive immune response would eliminate the virus.
Our gene expression studies revealed a number of interesting correlations. First, MxA was highly induced after viral infection in control cells but was hardly detectable in any of the five glioblastoma cell lines. When pretreated with IFN-α or poly(I:C), four out of five glioblastoma cell lines responded with a small induction, compared to control cells that had a significantly greater response. It is of interest to note that although the complete protection of control cells is associated with an MxA gene induction of up to 1,000-fold, the only glioblastoma cell type that was somewhat protected by IFN-α pretreatment, A-172, did not express MxA to any significant levels. Although MxA has been primarily associated with myxovirus resistance, evidence exists for a role for MxA in the IFN-mediated inhibition of VSV (31, 35). However, the requirement for MxA in the antiviral defense against VSV may differ in tumor and normal brain cells. IFN-β expression was very strong in control cells upon VSV-rp30a infection but was lacking after poly(I:C) treatment. In tumor cells, on the other hand, IFN-β mRNA was completely absent in two tumor cell types, U-87 and U-118. Interestingly, these tumor cells have the greatest sensitivity for VSV oncolysis but also display the fastest growth pattern in our experimental conditions. In that regard, it is of importance to note that though they were originally described as antiviral elements of the innate immune system, IFN-α/β also may play a role in control of cell differentiation and proliferation and in prevention of malignant transformation (8). Functional loss of IFN-α and -β appears to be common in the mutation repertoire of gliomas, especially in highly malignant glioblastoma multiforme (33), and the extent of IFN-α/β defects increases with malignant progression. A previous study revealed an intact IFN status in low-grade gliomas (WHO grade 2), hemizygous IFN deletions in anaplastic astrocytomas (grade 3), and a nullizygous IFN status in glioblastoma multiforme (grade 4) (15). However, expression of certain IFN-α/β-stimulated genes does not necessarily correlate with antiviral potential in glioblastoma cells against VSV. In fact, our studies on ISG15 gene expression revealed a slightly greater induction in tumor cells than in normal cells. ISG15 is a ubiquitin-like protein suggested to be involved in cellular protein modification. However, a role for ISG15 in antiviral defense is complex, as ISG15 appears to inhibit the replication of some viruses (human immunodeficiency virus type 1 and Sindbis virus) (16, 23) but has been shown to be dispensable for defense against lymphocytic choriomeningitis virus and VSV (24).
This study was designed to address the important issue of enhancing the safety of a potential application of VSV as an oncolytic agent in the brain. VSV has only recently been recognized as a potential tool to attack malignancies. A number of reports have demonstrated the oncolytic effects of VSV in several in vitro and in vivo tumor models (reviewed in references 3 and 17), and the importance of the IFN-α/β system as the first line of defense for normal cells outside the brain has been well established. Application of replication-competent oncolytic viruses in the brain, however, represents a particular challenge considering the special immune status of the CNS parenchyma. Glioblastoma brain tumors may be an attractive target for VSV-based oncolytic approaches. The molecular makeup of those tumors can be heterogeneous, making it difficult for viral vectors that are limited in targeting only specific mutations. For example, reovirus requires activated Ras signaling pathways, and oncolytic adenoviruses are based upon p53 defects (5). VSV on the other hand, selectively killed a number of glioblastoma types with different genetic aberrations but in the presence of IFN-α did not kill normal human astrocytes or mixed primary cultures from human brain. Importantly, as the time-lapse video experiments showed, even with a potentially increasing virus titer during infection and VSV replication in glioblastoma cells, the normal astrocytes in the same culture dish were continuously protected by a single application of IFN or poly(I:C) at the beginning of the 4-day time course of the experiment.
Another consideration is the size and invasive nature of glioblastomas, which are two of the main factors why the outcome of previous clinical trials with replication-incompetent vectors has been disappointing (reviewed in reference 27). VSV rapidly replicates and has been shown in a previous study from our lab to spread through the whole tumor mass of U-87 xenografts with minimal infection of surrounding host tissue (42), indicating potential for application in a widespread and invasive glioblastoma mass. However, uncontrolled spread of the virus, particularly in the brain, would result in deleterious consequences. We show here that IFN-α/β as well as an inducer of the IFN-α/β pathway can protect normal human brain cells from VSV infection in vitro. It is important to note that intravenous IFN-β is an approved treatment modality for severe multiple sclerosis cases, indicating effective and safe action of this group of drugs within the brain. Moreover, IFN-α/β has also been shown to be effective against glioma directly through apoptosis induction (44) and indirectly through inhibition of angiogenesis (26). In our experimental study, we successfully combined an oncolytic VSV with IFN, which not only may result in improved safety but also may act synergistically against glioma.
As the protective effects of IFN on normal brain cells but not on glioblastoma cells were found with both VSV variants used here, it is likely that these results may also generalize to other VSV strains that also may possess oncolytic potential (17, 18, 21). In addition, IFN can also protect brain cells against other unrelated viruses. For example, IFN-α, -β, and -γ protect the developing brain from cytomegalovirus infection (40). The antiviral effect was significantly stronger against VSV than against cytomegalovirus, further supporting the view that IFN sensitivity of VSV can serve as a potential safety measure. Additionally, the data here may also prove useful in devising strategies for protecting normal brain, but not brain tumors, during treatment with oncolytic strains of herpesvirus, adenovirus, reovirus, and other replication-competent viruses (5, 27).
Acknowledgments
We thank Prabhat Ghosh and Koray Ozduman for construction and generation of red fluorescent U-87 cells and Prasanthi Bandi for technical assistance.
Support for this work was provided by NIH grants K22 AI064757 (to M.D.R.) and AI48854 (to A.N.V.D.P.).
Footnotes
Published ahead of print on 15 November 2006.
REFERENCES
- 1.Ahmed, M., S. D. Cramer, and D. S. Lyles. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 330:34-49. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran, S., M. Porosnicu, and G. N. Barber. 2001. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J. Virol. 75:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber, G. N. 2005. VSV-tumor selective replication and protein translation. Oncogene 24:7710-7719. [DOI] [PubMed] [Google Scholar]
- 4.Chesler, D. A., and C. S. Reiss. 2002. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 13:441-454. [DOI] [PubMed] [Google Scholar]
- 5.Chiocca, E. A. 2002. Oncolytic viruses. Nat. Rev. Cancer 2:938-950. [DOI] [PubMed] [Google Scholar]
- 6.Colton, C. A., J. Yao, J. E. Keri, and D. Gilbert. 1992. Regulation of microglial function by interferons. J. Neuroimmunol. 40:89-98. [DOI] [PubMed] [Google Scholar]
- 7.Connor, J. H., C. Naczki, C. Koumenis, and D. S. Lyles. 2004. Replication and cytopathic effect of oncolytic vesicular stomatitis virus in hypoxic tumor cells in vitro and in vivo. J. Virol. 78:8960-8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dafny, N., and P. B. Yang. 2005. Interferon and the central nervous system. Eur. J. Pharmacol. 523:1-15. [DOI] [PubMed] [Google Scholar]
- 9.Dalton, K. P., and J. K. Rose. 2001. Vesicular stomatitis virus glycoprotein containing the entire green fluorescent protein on its cytoplasmic domain is incorporated efficiently into virus particles. Virology 279:414-421. [DOI] [PubMed] [Google Scholar]
- 10.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17:251-263. [DOI] [PubMed] [Google Scholar]
- 11.Duntsch, C. D., Q. Zhou, H. R. Jayakar, J. D. Weimar, J. H. Robertson, L. M. Pfeffer, L. Wang, Z. Xiang, and M. A. Whitt. 2004. Recombinant vesicular stomatitis virus vectors as oncolytic agents in the treatment of high-grade gliomas in an organotypic brain tissue slice-glioma coculture model. J. Neurosurg. 100:1049-1059. [DOI] [PubMed] [Google Scholar]
- 12.Ebert, O., S. Harbaran, K. Shinozaki, and S. L. Woo. 2005. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 12:350-358. [DOI] [PubMed] [Google Scholar]
- 13.Farina, C., M. Krumbholz, T. Giese, G. Hartmann, F. Aloisi, and E. Meinl. 2005. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J. Neuroimmunol. 159:12-19. [DOI] [PubMed] [Google Scholar]
- 14.Ichinohe, T., I. Watanabe, S. Ito, H. Fujii, M. Moriyama, S. Tamura, H. Takahashi, H. Sawa, J. Chiba, T. Kurata, T. Sata, and H. Hasegawa. 2005. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 79:2910-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James, C. D., J. He, E. Carlbom, M. Nordenskjold, W. K. Cavenee, and V. P. Collins. 1991. Chromosome 9 deletion mapping reveals interferon alpha and interferon beta-1 gene deletions in human glial tumors. Cancer Res. 51:1684-1688. [PubMed] [Google Scholar]
- 16.Lenschow, D. J., N. V. Giannakopoulos, L. J. Gunn, C. Johnston, A. K. O'Guin, R. E. Schmidt, B. Levine, and H. W. Virgin IV. 2005. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J. Virol. 79:13974-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichty, B. D., A. T. Power, D. F. Stojdl, and J. C. Bell. 2004. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol. Med. 10:210-216. [DOI] [PubMed] [Google Scholar]
- 18.Lichty, B. D., D. F. Stojdl, R. A. Taylor, L. Miller, I. Frenkel, H. Atkins, and J. C. Bell. 2004. Vesicular stomatitis virus: a potential therapeutic virus for the treatment of hematologic malignancy. Hum. Gene Ther. 15:821-831. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza-Fernandez, V., R. D. Andrew, and C. Barajas-Lopez. 2000. Interferon-alpha inhibits long-term potentiation and unmasks a long-term depression in the rat hippocampus. Brain Res. 885:14-24. [DOI] [PubMed] [Google Scholar]
- 20.Morrey, J. D., C. W. Day, J. G. Julander, L. M. Blatt, D. F. Smee, and R. W. Sidwell. 2004. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir. Chem. Chemother. 15:101-109. [DOI] [PubMed] [Google Scholar]
- 21.Obuchi, M., M. Fernandez, and G. N. Barber. 2003. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 77:8843-8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohgaki, H. 2005. Genetic pathways to glioblastomas. Neuropathology 25:1-7. [DOI] [PubMed] [Google Scholar]
- 23.Okumura, A., G. Lu, I. Pitha-Rowe, and P. M. Pitha. 2006. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc. Natl. Acad. Sci. USA 103:1440-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osiak, A., O. Utermohlen, S. Niendorf, I. Horak, and K. P. Knobeloch. 2005. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus. Mol. Cell. Biol. 25:6338-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlovic, J., H. A. Arzet, H. P. Hefti, M. Frese, D. Rost, B. Ernst, E. Kolb, P. Staeheli, and O. Haller. 1995. Enhanced virus resistance of transgenic mice expressing the human MxA protein. J. Virol. 69:4506-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puduvalli, V. K., and R. Sawaya. 2000. Antiangiogenesis—therapeutic strategies and clinical implications for brain tumors. J. Neurooncol. 50:189-200. [DOI] [PubMed] [Google Scholar]
- 27.Pulkkanen, K. J., and S. Yla-Herttuala. 2005. Gene therapy for malignant glioma: current clinical status. Mol. Ther. 12:585-598. [DOI] [PubMed] [Google Scholar]
- 28.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1244. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 29.Salazar, A. M., H. B. Levy, S. Ondra, M. Kende, B. Scherokman, D. Brown, H. Mena, N. Martin, K. Schwab, D. Donovan, D. Dougherty, M. Pulliam, M. Ippolito, M. Graves, H. Brown, and A. Ommaya. 1996. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery 38:1096-1103. [PubMed] [Google Scholar]
- 30.Satoh, J., and Y. Kuroda. 2001. Differing effects of IFN beta vs IFN gamma in MS: gene expression in cultured astrocytes. Neurology 57:681-685. [DOI] [PubMed] [Google Scholar]
- 31.Schanen, C., V. Chieux, P. E. Lobert, J. Harvey, and D. Hober. 2006. Correlation between the anti-virus-induced cytopathic effect activity of interferon-alpha subtypes and induction of MxA protein in vitro. Microbiol. Immunol. 50:19-24. [DOI] [PubMed] [Google Scholar]
- 32.Scumpia, P. O., K. M. Kelly, W. H. Reeves, and B. R. Stevens. 2005. Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia 52:153-162. [DOI] [PubMed] [Google Scholar]
- 33.Sehgal, A. 1998. Molecular changes during the genesis of human gliomas. Semin. Surg. Oncol. 14:3-12. [DOI] [PubMed] [Google Scholar]
- 34.Shinozaki, K., O. Ebert, and S. L. Woo. 2005. Eradication of advanced hepatocellular carcinoma in rats via repeated hepatic arterial infusions of recombinant VSV. Hepatology 41:196-203. [DOI] [PubMed] [Google Scholar]
- 35.Staeheli, P., and J. Pavlovic. 1991. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J. Virol. 65:4498-4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263-275. [DOI] [PubMed] [Google Scholar]
- 37.Tazulakhova, E. B., O. V. Parshina, T. S. Guseva, and F. I. Ershov. 2001. Russian experience in screening, analysis, and clinical application of novel interferon inducers. J. Interferon Cytokine Res. 21:65-73. [DOI] [PubMed] [Google Scholar]
- 38.Tedeschi, B., J. N. Barrett, and R. W. Keane. 1986. Astrocytes produce interferon that enhances the expression of H-2 antigens on a subpopulation of brain cells. Cell Biol. 102:2244-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trottier, M. D., Jr., B. M. Palian, and C. Shoshkes Reiss. 2005. VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology 333:215-225. [DOI] [PubMed] [Google Scholar]
- 40.van den Pol, A. N., M. D. Robek, P. K. Ghosh, K. Ozduman, P. Bandi, M. D. Whim, and G. Wollmann. 2007. Cytomegalovirus induces interferon-stimulated gene expression and is attenuated by interferon in the developing brain. J. Virol. 81:332-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watkins, L. R., S. F. Maier, and L. E. Goehler. 1995. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 57:1011-1026. [DOI] [PubMed] [Google Scholar]
- 42.Wollmann, G., P. Tattersall, and A. N. van den Pol. 2005. Targeting human glioblastoma cells: comparison of nine viruses with oncolytic potential. J. Virol. 79:6005-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada, T., M. A. Horisberger, N. Kawaguchi, I. Moroo, and T. Toyoda. 1994. Immunohistochemistry using antibodies to alpha-interferon and its induced protein, MxA, in Alzheimer's and Parkinson's disease brain tissues. Neurosci. Lett. 181:61-64. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida, J., M. Mizuno, and T. Wakabayashi. 2004. Interferon-beta gene therapy for cancer: basic research to clinical application. Cancer Sci. 95:858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]