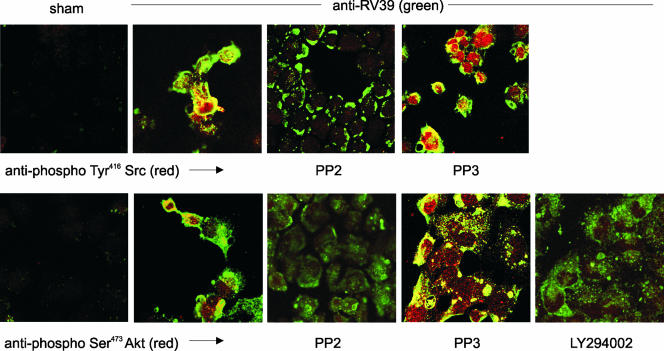
FIG. 2.
RV39 internalization and colocalization with phospho-Tyr416 Src (upper panel) and phospho-Ser473Akt (lower panel) are blocked by chemical Src inhibitors. 16HBE14o− cells were incubated with sham or RV39 at an MOI of 100. RV39 (shown in green) induced Tyr416 phosphorylation of Src (red, upper panel) and colocalized with Src (yellow). The high-affinity Src inhibitor PP2 blocked pTyr416 Src colocalization of RV but did not block virus binding. The low-affinity inhibitor PP3 did not block the association of RV and Src. RV39 also induced Ser473 phosphorylation of Akt (red, lower panel) and colocalized with an Akt fraction (yellow). PP2 appeared to block Ser473 Akt phosphorylation and the colocalization of RV and Akt. The low-affinity inhibitor PP3 did not block the association of RV and Akt. LY294002 also appeared to block virus internalization and colocalization with phospho-Ser473Akt.