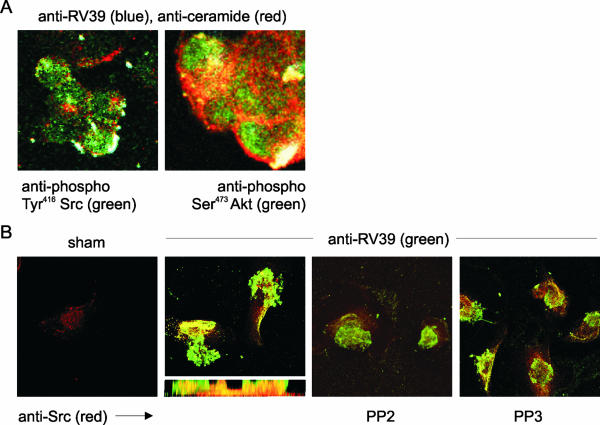
FIG. 5.
Digoxigenin-labeled RV39 colocalizes with phosphorylated Src and Akt in ceramide-containing lipid rafts. (A) RV39 immunoreactivity (blue) colocalized with ceramide (red), phospho-Tyr416 Src (green, left panel), and phospho-Ser473 Akt (green, right panel) in a white merged image. The colocalization of both Src and Akt with ceramide in punctate structures at the cell surface suggests that these protein kinases are associated with raft complexes where bound RV is aggregated. (B) Digoxigenin-labeled RV39 also colocalized with Src in primary mucociliary differentiated airway epithelial cells. The apical surface of primary airway epithelial cells grown at an air-liquid interface were incubated with sham or digoxigenin-labeled RV39 at an MOI of 100. Digoxigenin-labeled RV39 (shown in green) localized to the airway epithelial cell surface. Src was visualized using the clone GD11 antibody (shown in red). RV39 appeared to colocalize with Src just under the plasma membrane (see z-axis section). The high-affinity Src inhibitor PP2 appeared to block the colocalization of RV and Src. The low-affinity inhibitor PP3 did not block the association of RV and Src.