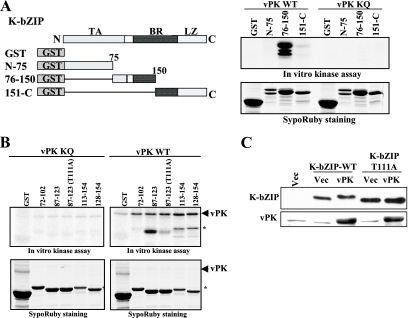
FIG. 6.
Mapping of vPK phosphorylation site of K-bZIP. (A) The domains of K-bZIP and the GST-K-bZIP mutants are indicated in the left panel. TA, transactivation domain; BR, basic domain; LZ, leucine zipper domain. Results of the in vitro protein kinase assay and SyproRuby staining of the same gel are shown at right. (B) The coding region of recombinant K-bZIP is indicated at the top of the panels. Results of the in vitro protein kinase assay and SyproRuby staining of the same gels are shown. Asterisks show phosphorylated protein originated from E. coli. (C) In vivo phosphorylation. The K-bZIP-wild type (WT) or K-bZIP-T111A expression plasmid was cotransfected with the indicated plasmids into 293 cells. K-bZIP or vPK was probed with specific antibody. The molecular weight of K-bZIP wild type but not K-bZIP-T111A was increased when K-bZIP was cotransfected with vPK. WB, Western blotting; Vec, vector; vPK KQ, vPK with the mutation K108Q.