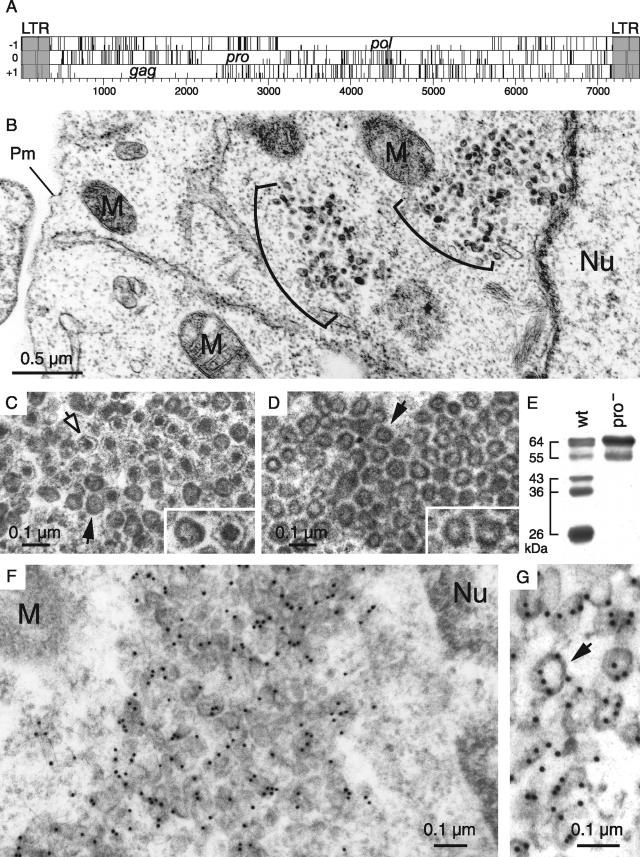
FIG. 1.
Structure of the MusD genome and associated VLPs. (A) Genomic organization of autonomous MusD elements, with the LTRs (gray) bordering the three ORFs homologous to the retroviral gag, pro, and pol genes. No ORF for an envelope protein can be identified. (B to D) Electron microscopy of the VLPs encoded by the retrotransposition competent MusD-6 copy (B and C) and a MusD-6 protease mutant (D), observed in human cells 48 h after transfection. (B) Representative low-magnification image of a transfected HeLa cell, with numerous particles organized in intracellular clusters. The nucleus (Nu), mitochondria (M), and plasma membrane (Pm) are indicated. No particle can be observed at the level of the cell membrane or in the extracellular space. (C) High-magnification view of a cluster of particles with two different morphologies: immature (solid arrow) with an electron-lucent core and mature (open arrow) with a condensed central core. (D) Same magnification as in panel C for a cluster of particles observed in cells transfected with a protease-deficient MusD-6 mutant. Only immature particles can be observed (arrow). (E) Western blot analysis of whole-cell lysates of HeLa cells transfected with the MusD-6 copy (wt, lane 1) or the protease-deficient mutant (pro−, lane 2) used in panels B to D. The proteins were separated by SDS-PAGE, and Gag products were revealed using a rabbit antiserum directed against a MusD Gag recombinant protein (30). The molecular masses of the Gag precursor and protein cleavage products are indicated in kilodaltons. (F and G) Immunogold labeling of MusD particles in transfected cells using the anti-Gag MusD antiserum and a secondary antibody linked to gold beads, observed by electron microscopy; labeling is seen within the clusters (F), at the level of the MusD particles (a higher-magnification view is seen in panel G [see arrow]).