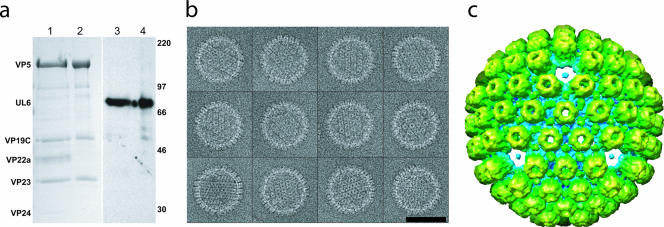
FIG. 1.
Urea-extracted capsids. (a) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis results for control (lanes 1 and 3) and 6 M urea-treated (lanes 2 and 4) capsids. Coomassie blue staining (lanes 1 and 2) shows that the urea-treated capsid sample contains the hexon (VP5) and triplex (VP19C, VP23) proteins but has lost the scaffolding protein (VP22a) and protease (VP24). The reduction in VP5 levels due to the loss of pentons is too small (6%) to be evident on this gel. Western blotting with MAb175 (lanes 3 and 4) (14) confirms that the portal protein (UL6) is not removed by urea extraction. Size markers are indicated in kilodaltons on the right. (b) A gallery of boxed capsids treated with 6 M urea. Bars, 100 nm. (c) A 3-nm resolution single-particle icosahedral reconstruction looking along a threefold orientation shows the capsid lacking pentons.