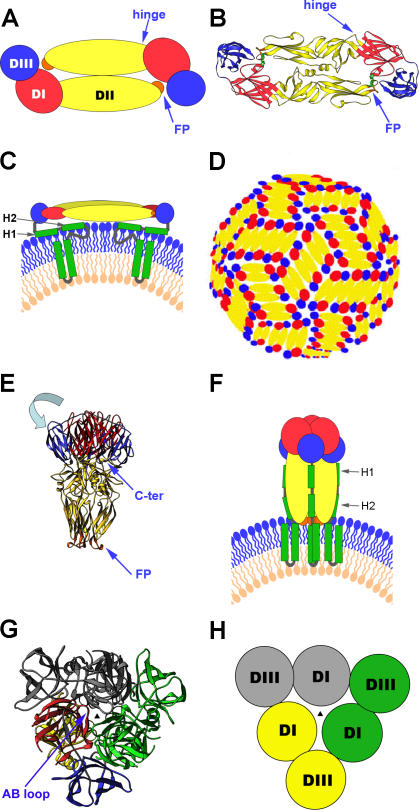
Figure 1. Summary of the Structural Organization and Different Conformations of the Flavivirus Envelope Protein E.
(A) Schematic top view of the organization of the sE protein dimer as present at the surface of mature virions, color-coded according to the three domains (DI, DII, and DIII). The FP is indicated in orange.
(B) Crystal structure (top view) of the TBE virus sE dimer [31] represented as a ribbon diagram.
(C) Schematic side view of the E dimer at the surface of mature virions, with the “stem” and TM C-terminal polypeptide segments (missing in the truncated sE form) indicated in green. The viral lipid bilayer is illustrated with lipids belonging to the outer and inner leaflets colored blue and pink, respectively. Cryoelectron microscopy 3D reconstructions have shown that the stem forms two α-helices (H1 and H2) lying on the viral membrane, followed by the two TM segments [37].
(D) Schematic diagram illustrating the icosahedral arrangement of E dimers at the surface of mature flavivirus particles—in a “herringbone” pattern—as determined for dengue and West Nile virus [29,30]. Ninety E dimers form a rigid glycoprotein cage enclosing the viral membrane.
(E) Structure of the TBE virus sE in its trimeric postfusion conformation [33], represented as a ribbon diagram. Compared to the structure of E in the prefusion dimer, DIII is translocated (in a movement indicated by the light-blue arrow) to a lateral position, with its C terminus (labeled C-ter) projecting toward the FPs, thus generating a hairpin-like conformation.
(F) Schematic representation illustrating the proposed organization of full-length E in its postfusion conformation. In this model, the α-helices of the stem interact with the body of the trimer, in the grooves between adjacent, parallel DIIs. The lipid bilayer as well as the stem and TM segments are drawn as in (C).
(G) Top view of the sE trimer. For clarity, one of the subunits is colored according to domains, and the other two are given in a single color each (green and gray). The AB loop of DI (labeled in the figure [G]) rearranges upon dislocation of DIII, to make most of the DI–DI trimeric contacts. The relocated DIII acts as an external clamp, inserting into the grooves between DIs and providing additional intersubunit contacts. The vertical 3-fold axis at the center is indicated by a black triangle.
(H) Schematic drawing to simplify the top view of the sE trimer, matching the color coding of (G) (except for the subunit in yellow, which corresponds to the one colored by domains in [G]), to highlight the trimer-stabilizing role of DIII in the hairpin-like conformation of the molecule.