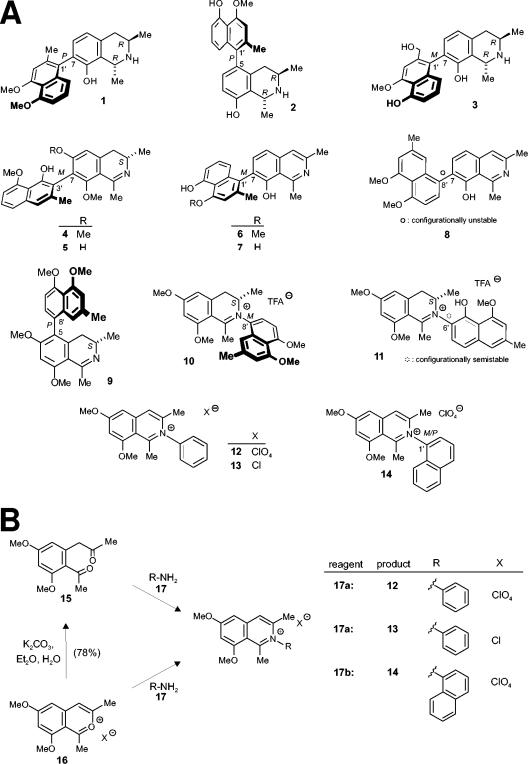
FIG. 1.
Substances tested for activity against L. major. (A) Structures of the naphthylisoquinoline alkaloids dioncophyllines A (compound 1) and C (compound 2), dioncopeltine A (compound 3), ancistrocladidine (compound 4), ancistroheynine B (compound 5), ent-dioncophylleine A (compound 6), 5′-O-demethyl-ent-dioncophylleine A (compound 7), dioncophylleine D (compound 8), ancistroealaine A (compound 9), ancistrocladiniums A (compound 10) and B (compound 11), and the synthetically prepared analogs (compounds 12 to 14). Note that the naphthylisoquinolinium salt (compound 14) occurs as a racemic mixture of atropisomers at the rotationally hindered N,C axis. (B) Synthesis of naphthylisoquinolinium salts 12 to 14. Naphthylisoquinoline alkaloid analogs compounds 12, 13, and 14) were synthesized from compounds 15 and 16. TFA, trifluoroacetic acid.