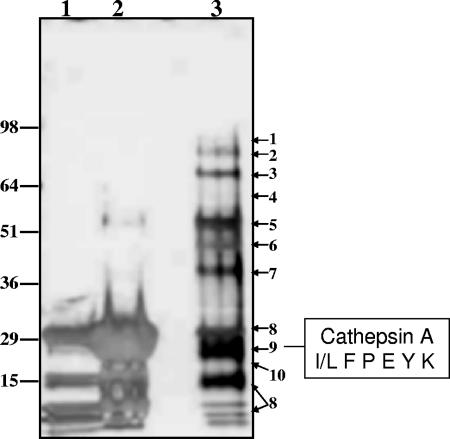
FIG. 3.
SDS-PAGE resolution and identification of proteins in the ConA pool. Proteins present in the ConA pool were concentrated to 60 μl and resolved using SDS-PAGE (4 to 20% acrylamide gradient gel, NUPAGE; Invitrogen, Inc., Carlsbad, CA). Lanes: 1, ConA beads after elution; 2, ConA beads eluted with 8 M urea; 3, concentrated ConA pool (10 μl). Protein bands in the 10-μl lane were visualized using silver staining. Adjacent areas of the gel in an unstained lane (50 μl aliquot loaded) corresponding to a stained protein band were excised and identified by MS-MS sequencing of tryptic peptides generated by digestion. The resultant peptides were purified by passage through a C18 ZipTip column, analyzed by positive ESI-MS, and sequenced using MS-MS fragmentation. Proteins were identified from the generated sequences by BLAST analysis of the NCBI nonredundant protein/peptide database (1). Proteins indicated to the right: 1, fibrinogen; 2, N-acetylglucosamidase; 3, acid α-glucosidase; 4, sulfamidase; 5, fibrinogen; 6, α-fucosidase; 7, iduronate sulfatase; 8, concanavalin A; 9, CatA; 10, cathepsin Z/X.