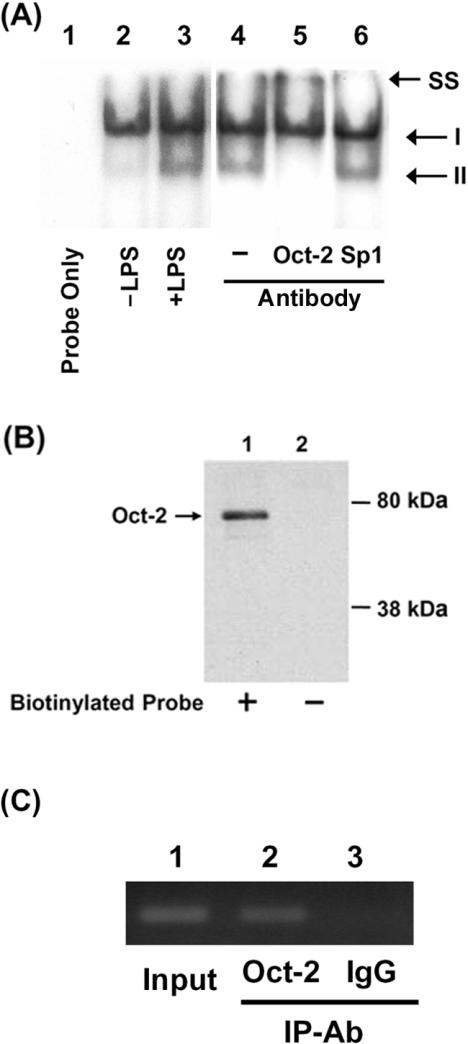
Figure 4. Binding of Oct-2 to the Octamer element in the resistin promoter.
Binding of Oct-2 to the Octamer element was demonstrated by EMSA (A), DNA-affinity precipitation assay (B) and ChIP (C). (A) EMSAs were carried out as described in the Experimental section using a probe covering nucleotides −927 to −895 of the resistin promoter. Lane 1, probe alone; lane 2, 20 μg of nuclear extract from untreated cells; lanes 3–6, 20 μg of nuclear extract from LPS-treated cells; lane 4, no Ab; lane 5, Oct-2 Ab; lanes 6, Sp1 Ab. SS, supershift. (B) Nuclear protein (100 μg) from LPS-treated cells (lane 1) was incubated with a biotinylated oligonucleotide from the Octamer region (−927 to −895 bp) of the resistin promoter, and the complex captured using streptavidin-coated magnetic beads. The captured proteins were separated by SDS/8% PAGE and immunoblotted for Oct-2. Nuclear extracts incubated without the biotinylated oligonucleotide were used as negative controls (lanes 2). (C) RAW264.7 cells were treated with LPS (10 ng/ml) for 12 h, then ChIP assays were performed as described in the Experimental section. IgG was used as a negative control. A 299 bp resistin promoter (−1193 to −895 bp) was amplified by PCR and separated on an agarose gel. A total of 10% of the chromatin DNA used for immunoprecipitation (IP) was subjected to PCR and is indicated as ‘Input’.