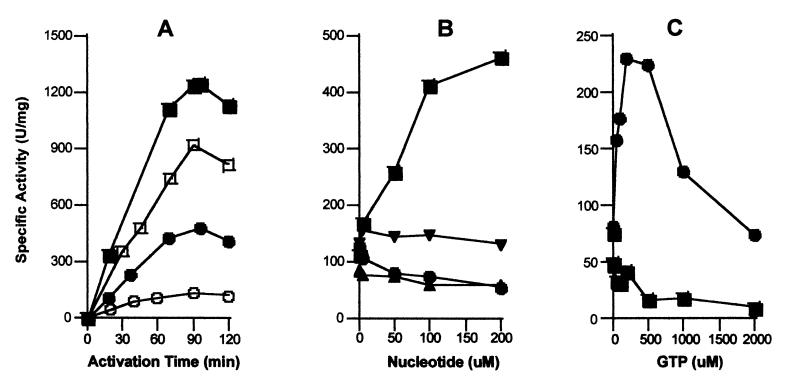
Figure 3.
GTP-dependence of urease apoprotein activation for the UreD-UreF-UreG-apourease complex. (A) The time dependence of urease apoprotein activation using UreD-UreF-UreG-apourease (0.2 μM) was studied at 37°C in the presence (closed symbols) or absence (open symbols) of 200 μM Mg2GTP in 100 mM Hepes buffer (pH 8.3) containing 150 mM NaCl, 100 μM NiCl2, and 100 mM (squares) or 100 μM (circles) bicarbonate. (B) The nucleotide specificity and concentration dependence was examined by monitoring the extent of activation after 1.5 h in buffer containing 100 μM NiCl2, 100 μM NaHCO3, and the indicated concentrations of Mg2GTP (■), Mg2ATP (▴), Mg2GDP (●), and Mg2GMP-PNP (▾). (C) The effect of using a P-loop defective UreG variant (T21A UreG) was assessed. The urease apoproteins in UreD-UreF-UreG-apourease complexes containing the variant protein (0.17 μM; ■) or native UreG (0.2 μM; ●) were activated for 1.5 h in the presence of 100 μM NiCl2, 100 μM NaHCO3, and GTP at the concentrations indicated. In all cases, urease activities were measured as previously described. All results are representative of three separate protein preparations.