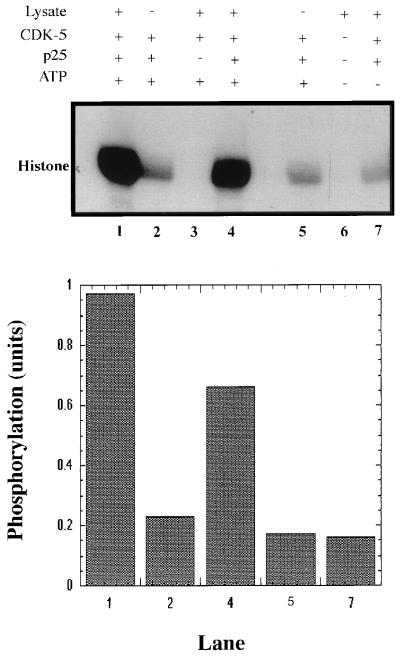
Figure 2.
Effect of phosphorylation of cdk5 on its catalytic activity. Equal amounts of GST-cdk5 and a molar excess of GST-p25 were incubated with (+) or without (−) PC12 lysate in the presence (+) or absence (−) of 2 mM ATP (nonradioactive) at 30°C for 1 h. GST-cdk5 samples in lanes 1, 2, and 7 were preincubated with GST-p25 for 3 h and were not preincubated in lanes 4 and 5. Control experiments in the absence of GST-p25 (lane 3) and in the absence of GST-cdk5/GST-p25 (lane 6) also were performed. The fusion proteins were recovered on glutathione-Sepharose beads, washed, and assayed for histone kinase activity. The radiolabeled products were electrophoresed and detected by autoradiography. (Upper) Representative of three experiments. (Lower) The quantitation of phosphorylation by densitometric analyses of the corresponding lanes in Upper.