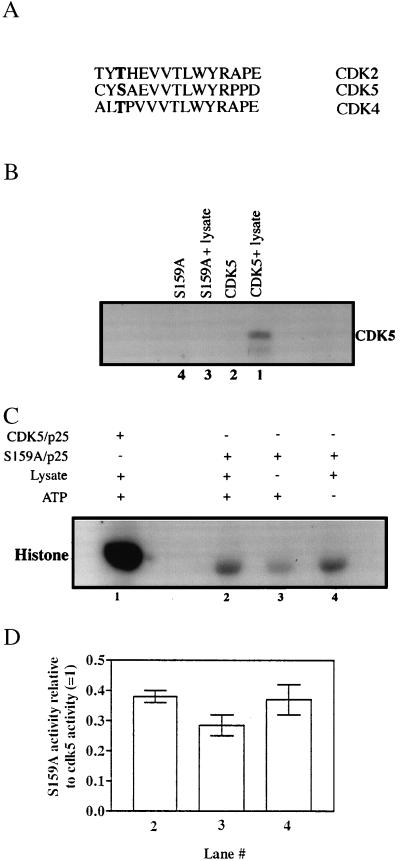
Figure 4.
(A) Alignment of the activation-loop residues of various cdk kinases showing conservation of the phosphorylatable regulatory residue in boldface. (B) Effect on cdk5 phosphorylation of mutation of Ser159 to Ala. Equal amounts of GST-cdk5 (lane 1) or GST-S159A (lane 3) mutant were subjected to phosphorylation by the PC12 cell lysate and the modified proteins were recovered as described in Fig. 1. The GST-fusion tags were cleaved with thrombin and clarified by using glutathione-Sepharose beads before SDS/PAGE analysis. The radiolabeled products were detected by autoradiography. (C) Histone kinase activity of S159A. Equal amounts of GST-cdk5 or GST-S159A were preincubated with GST-p25 (molar excess) for 3 h before incubation with (lanes 1 and 2) or without (lane 3) PC12 cell lysate in presence (+) or absence (−) of nonradioactive ATP. Glutathione-Sepharose beads were used to separate the fusion proteins and used to phosphorylate histone. The radiolabeled histone was detected by autoradiography. Lane 1 is the same experiment done with wild-type GST-cdk5/p25 under similar conditions. (D) Quantitation of the data for experiments described in Fig. 4C by densitometric analyses of the 32P-labeled histone bands. The histogram represents the average of results obtained from two independent experiments (shown as bars) expressed relative to the activity of wild-type cdk5/p25. Lane numbers correspond to those in Fig. 4C. Statistical analysis by using the two-tailed t test did not reveal any significant differences between the data for lanes 2 and lane 4 vs. lane 3 (P > 0.1 and P > 0.2, respectively). The activity of wild-type cdk5 was taken as 1 (lane 1).