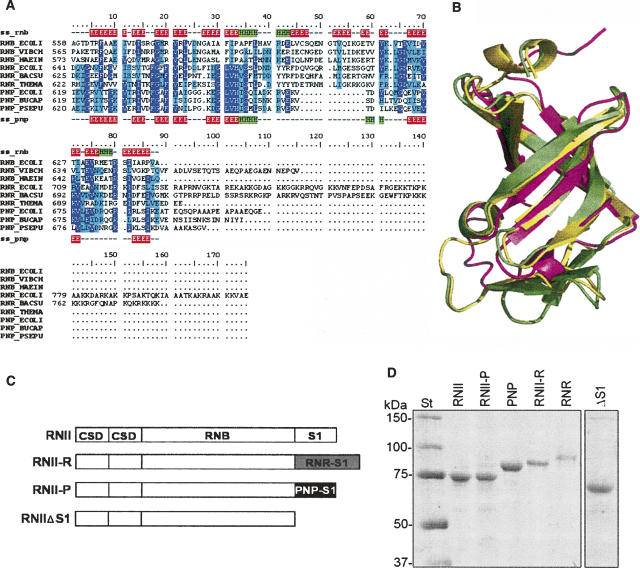
FIGURE 1.
(A) A structure-based multiple sequence alignment of S1 domains from RNase II (RNB), RNase R (RNR), and PNPases (PNP) of several organisms (ECOLI: Escherichia coli; VIBCH: Vibrio cholerae; HAEIN: Haemophilus influenzae; BACSU: Bacillus subtilis; THEMA: Thermotoga maritima; BUCAP: Buchnera aphidicola ; PSEPU: Pseudomonas putida). Alignment is colored according to conservation: highly conserved residues are in purple, semiconserved residues are in cyan. Secondary structure elements of S1 domains from RNB_ECOLI (ss_rnb) and PNP_ECOLI (ss_pnp) are also indicated: β-sheets are in red and α-helices are in green. (B) A structural alignment of experimentally determined structures for the S1 domains of E. coli RNase II (yellow; PDB code: 2IX0) (Frazão et al. 2006) and PNPase (magenta; PDB code: 1SRO) (Bycroft et al. 1997) and a homology model generated for the S1 domain of E. coli RNase R (green). (C) A schematic representation of the domain organization of RNase II (RNII), RNase II-R (RNII-R), RNase II-P (RNII-P), and truncated protein RNase IIΔS1 (RNIIΔS1). Protein domains from RNase II (CSD, RNB, and S1) are represented as white rectangles, the S1 domain from RNase R is represented as a gray rectangle, and the S1 domain from PNPase as a black rectangle. (D). Purity of the enzymes was analyzed in a 10% SDS-PAGE; 0.5 μg of purified (His)6–RNase II (RNII), (His)6–RNase II-P (RNII-P), (His)6–PNPase (PNP), (His)6–RNase II-R (RNII-R), (His)6–RNase R (RNR), and (His)6–RNase IIΔS1 (ΔS1) were applied and visualized by Coomassie blue staining. The molecular weights of the standard proteins (St) are indicated on the left.