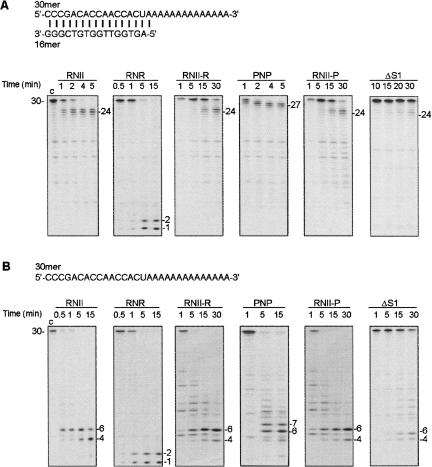
FIGURE 3.
Exoribonucleolytic activity of wild-type and hybrid proteins was assayed on a (A) double-stranded (16–30 ds) or (B) single-stranded substrate (30 ss). The enzyme concentrations used in A were: 0.5 nM of (His)6–RNase II (RNII), 0.5 nM of (His)6–RNase R (RNR), 60 nM of (His)6–RNase II-R (RNII-R), 0.5 nM of (His)6–PNPase (PNP), 200 nM of (His)6–RNase II-P (RNII-P), and 500 nM of (His)6–RNase IIΔS1 (ΔS1). The enzyme concentrations used in B were: 0.5 nM of (His)6–RNase II (RNII), 0.5 nM of (His)6–RNase R (RNR), 60 nM of (His)6–RNase II-R (RNII-R), 2 nM of (His)6–PNPase (PNP), 200 nM of (His)6–RNase II-P (RNII-P), and 50 nM of (His)6–RNase IIΔS1 (ΔS1). Reactions were performed as described in Materials and Methods and samples were withdrawn at the time points indicated in the figure. Reaction products were analyzed in a 20% PAA/7M urea gel and the bands detected by autoradiography. Substrate and product length are indicated. Control reactions, “c,” were incubated for 15 min with no enzyme added. The sequences of the oligoribonucleotides substrate used are depicted.