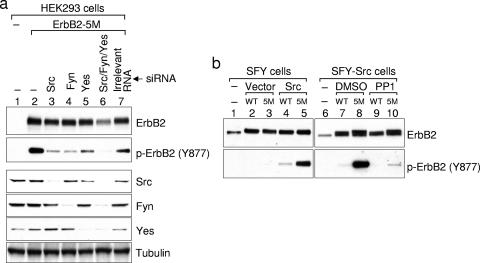
FIG. 3.
All three ubiquitously expressed Src kinases are involved in ErbB2 phosphorylation on Tyr877. (a) Reducing the expression of Src kinases by siRNA decreases ErbB2 Tyr877 phosphorylation. HEK-293 cells in six-well plates were transfected with siRNAs specific for Src, Fyn, or Yes using Lipofectamine 2000, followed by the transfection of ErbB2-5M plasmid with FuGene. On the third day after transfection, cells were lysed with modified RIPA buffer and total cell lysates were subjected to Western blotting analysis. Individual blots were probed for phosphorylated Tyr877 and for ErbB2, Src, Fyn, and Yes expression. A Western blot for tubulin in the cell lysates is included as a loading control. (b) ErbB2 phosphorylation on Tyr877 is diminished in SFY cells and is rescued by the expression of Src. SFY cells were transfected with wild-type ErbB2 or ErbB2-5M, together with wild-type Src or empty vector. Two days after transfection, cells were lysed and subjected to Western blotting analysis. For SFY-Src cells, single transfection of wild-type ErbB2 or ErbB2-5M was performed. One hour before lysis, cells were treated with 10 μM PP1 or the same amount of vehicle (dimethyl sulfoxide [DMSO]). ErbB2 protein and phosphorylation were determined as described in the legend to Fig. 1c.