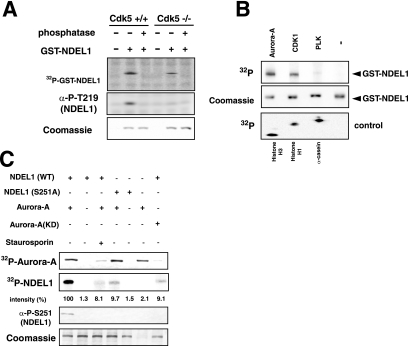
FIG. 1.
Aurora-A kinase phosphorylates Ser251 of NDEL1. (A) We performed in vitro phosphorylation using brain lysates from E15.5 wild-type mice or Cdk−/− mice (28). Phosphorylation was detected by the incorporation of 32P (upper panel) or by an anti-phospho-T219 (α-P-T219) monoclonal antibody (middle panel) (38). Phosphorylation at T219 was undetectable in lysates from Cdk−/− mice, but the lysate was competent to phosphorylate NDEL1 at other sites. Note that phosphatase removed this phosphorylation. (B) In vitro kinase assays using several mitotic kinases that are localized at the centrosome. CDK1 and Aurora-A efficiently phosphorylated NDEL1. Histone H3, histone H1, and casein were used as a positive control for each kinase. A control panel for protein loading is shown at the middle panel. (C) Examination of the effect of mutation at S251 or an Aurora-A inhibitor, staurosporin, on phosphorylation. Aurora-A(T288A) [Aurora-A(KD)] was used as a negative control of Aurora-A. The relative intensity of phosphorylation is described at the bottom. Phosphorylation was detected by the incorporation of 32P or an anti-phospho-S251 monoclonal antibody (see below). Either mutation or staurosporin significantly reduced phosphorylation. Note, an inactive form of Aurora-A, Aurora-A(KD), resulted in only background phosphorylation of NDEL1. −, absence of; +, presence of.