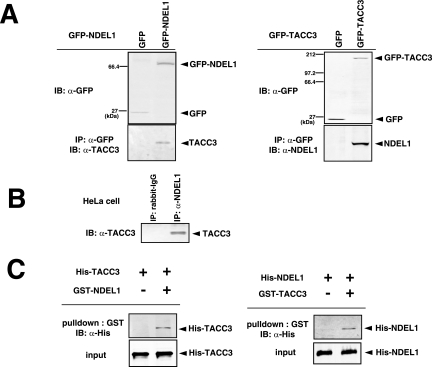
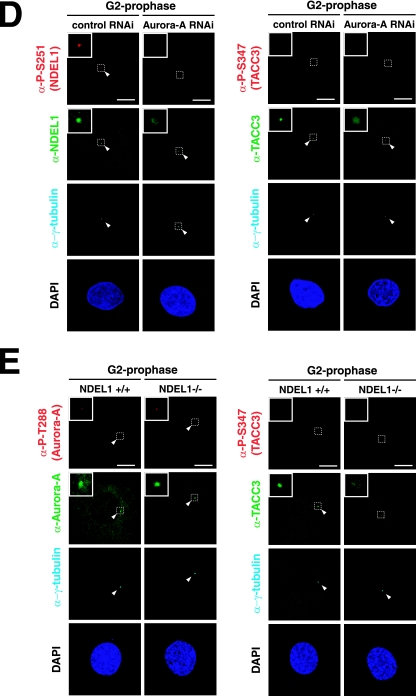
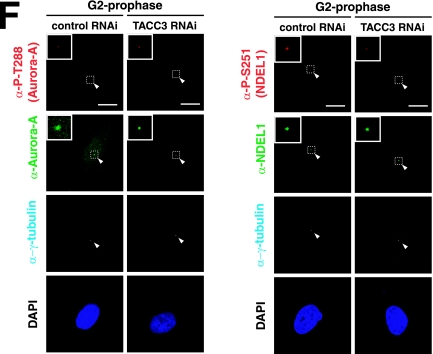
FIG.4.
Biochemical and genetic interaction between Aurora-A NDEL1 and TACC3. (A) Coimmunoprecipitation assays. HeLa cells were transfected with expression vectors as indicated. Immunoprecipitation (IP) was carried out using anti-GFP (α-GFP), and immunoblotting (IB) was performed with the anti-NDEL1 or anti-TACC3 antibody. Notice that the mobility of GFP-TACC3 is larger than the expected molecular mass from previous reports (19). (B) Coimmunoprecipitation assays using an anti-NDEL1 antibody. Endogenous NDEL1 clearly binds to TACC3. (C) GST pull-down. In vitro-translated HIS-NDEL1 and HIS-TACC3 were purified and incubated with purified GST-TACC3 and GST-NDEL, respectively, which were immobilized on glutathione-Sepharose 4B beads. Bound proteins were analyzed by Western blotting using an anti-HIS antibody. (D) Examination of phosphorylation and subcellular localization of NDEL1 and TACC3 in Aurora-A-depleted HeLa cells. Aurora-A-depleted HeLa cells exhibited dispersed distribution of NDEL1 and TACC3. The phosphorylation of both proteins was undetectable. (E) Examination of the phosphorylation and subcellular localization of Aurora-A and TACC3 in Ndel1-disrupted MEF cells. TACC3 appeared broadly distributed and lacked phosphorylation, whereas Aurora-A displayed phosphorylation at T288, suggesting that Aurora-A was activated despite the disruption of Ndel1 and that NDEL1 triggers centrosomal targeting of TACC3 and phosphorylation. (F) Examination of phosphorylation and subcellular localization of Aurora-A and NDEL1 in TACC3-depleted HeLa cells. Phosphorylation and subcellular localization of Aurora-A and NDEL1 appeared grossly normal despite the depletion of TACC3. RNAi, RNA interference; −, absence of; +, presence of.