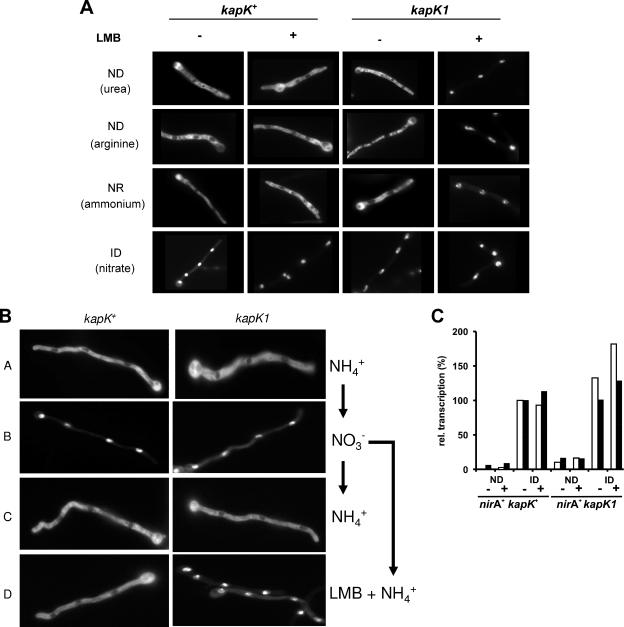
FIG. 2.
Nuclear export of NirA-GFP is mediated by KapK. (A) Mycelia from the wild-type (kapK+) and the kapK1 mutant strains were grown for 16 h at 25°C on coverslips in Aspergillus minimal medium under ND conditions (urea, arginine), NR conditions (ammonium), and ID conditions (nitrate). The effect of LMB on the subcellular localization of NirA was tested in the presence (+) or absence (−) of 10 ng of LMB/ml. (B) Analysis of the kapK-dependent nuclear export of NirA-GFP. Wild-type (kapK+) or kapK mutant (kapK1) cells were grown on coverslips in Aspergillus minimal medium containing ammonium (NH4+) as the sole nitrogen source for 16 h at 25°C (A). Nitrate (NO3−), was added to these cells and photographed after 2 min of incubation (B). Subsequently, the medium was withdrawn from the cultures and replaced again by ammonium-containing medium (NH4+) (C) or by medium containing ammonium and 10 ng of LMB/ml (LMB + NH4+) (D). Incubation proceeded for 10 min before the NirA-GFP localization was recorded. (C) Nuclear retention of NirA is not sufficient for transcriptional activation. Quantitative reverse transcription-PCR analysis of niiA (□) and niaD (▪) transcription in a nirA+ kapK+ strain was performed and compared to the LMB-sensitive nirA+ kapK1 mutant. Cultures were pregrown for 10 h on a noninducing-derepressing nitrogen source (urea), harvested, and shifted onto media containing urea (ND) or nitrate (ID) as the sole nitrogen source either in the absence (−) or in the presence (+) of 10 ng of LMB/ml. Incubation proceeded for 2 h before the cultures were harvested and analyzed. The actin gene (acnA) was used to normalize niiA and niaD expression. The transcriptional level of niiA and niaD in the nitrate-induced (ID) wild-type cultures in the absence of LMB (nirA+ kapK+, ID −) was set to 100%.