Abstract
The retromer is a cytosolic/peripheral membrane protein complex that mediates the retrieval of the cation-independent mannose 6-phosphate receptor from endosomes to the trans-Golgi network (TGN) in mammalian cells. Previous studies showed that the mammalian retromer comprises three proteins, named Vps26, Vps29, and Vps35, plus the sorting nexin, SNX1. There is conflicting evidence, however, as to whether a homologous sorting nexin, SNX2, is truly a component of the retromer. In addition, the nature of the subunit interactions and assembly of the mammalian retromer complex are poorly understood. We have addressed these issues by performing biochemical and functional analyses of endogenous retromers in the human cell line HeLa. We found that the mammalian retromer complex consists of two autonomously assembling subcomplexes, namely, a Vps26-Vps29-Vps35 obligate heterotrimer and a SNX1/2 alternative heterodimer or homodimer. The association of Vps26-Vps29-Vps35 with endosomes requires the presence of either SNX1 or SNX2, whereas SNX1/2 can be recruited to endosomes independently of Vps26-Vps29-Vps35. We also found that the presence of either SNX1 or SNX2 is essential for the retrieval of the cation-independent mannose 6-phosphate receptor to the TGN. These observations indicate that the mammalian retromer complex assembles by sequential association of SNX1/2 and Vps26-Vps29-Vps35 subcomplexes on endosomal membranes and that SNX1 and SNX2 play interchangeable but essential roles in retromer structure and function.
The retromer is a cytosolic/peripheral membrane protein complex that mediates retrograde transport of transmembrane cargo from endosomes to the Golgi complex (reviewed in references 4a and 35). This complex was originally described by S. Emr and colleagues for the yeast Saccharomyces cerevisiae, where it was found to comprise five subunits, named Vps5p, Vps17p, Vps26p, Vps29p, and Vps35p (Vps stands for vacuolar protein sorting) (19, 36, 37). Mutations in the genes encoding any of these subunits impair the retrieval of the vacuolar hydrolase receptor, Vps10p, the dibasic endopeptidase, Kex2p, and dipeptidyl aminopeptidase A from endosomes to the late Golgi complex, resulting in their rerouting to the vacuole, where they are degraded (19, 36, 37). As a consequence of Vps10p missorting, retromer-defective strains secrete vacuolar hydrolases into the periplasmic space (19, 29, 36).
Subsequent to their discovery in yeast, retromer subunit orthologs were found in all metazoans, including mammals (35). There are two orthologs of yeast Vps5p in mammals, namely, the sorting nexins SNX1 and SNX2 (16, 19). These proteins have a relatively unstructured amino-terminal segment followed by a PX domain that binds phosphatidylinositol 3-phosphate and other phosphoinositides (8, 12, 44) and a BAR domain that mediates dimerization and binding to highly curved membranes (6, 12, 43) (see scheme in Fig. 1). Yeast Vps17p has a similar domain organization, but there are no closely related homologs in mammals. Yeast Vps26p, Vps29p, and Vps35p all have at least one ortholog in mammals (Fig. 1), with which they share 30 to 42% sequence identity (17, 21). Like yeast Vps35p and Vps26p, the mammalian counterparts display no significant sequence identity with any other protein and no motifs that would provide insight into their functions. The yeast and mammalian Vps35 proteins interact with the cytosolic tails of Vps10p and its functional mammalian homolog, the cation-independent mannose 6-phosphate receptor (CI-MPR), respectively (1, 28); this led to the proposal that they function as the cargo recognition subunits of the corresponding retromer complexes. A recent X-ray crystallographic study has shown that the tertiary structure of Vps26 resembles that of the arrestins, suggesting that, like the arrestins, Vps26 could also participate in cargo recognition (38). Finally, other X-ray crystallographic analyses have shown that mammalian Vps29 has a phosphoesterase fold with a coordination site for two divalent metal cations. This fold is similar to those of a wide variety of prokaryotic and eukaryotic phosphoesterases (11, 42), and indeed, recombinant Vps29 in complex with Vps26 and Vps35 has been shown to have Zn2+-dependent phosphatase activity towards a serine-phosphorylated peptide derived from the cytosolic tail of the CI-MPR (13).
FIG. 1.
Schematic representation of mammalian retromer subunits. The number of amino acids and molecular mass of each protein are indicated. The scheme also indicates the features of the different subunits or their domains.
Despite the phylogenetic conservation of retromer subunits, the exact subunit composition and function of the mammalian retromer are not well established. SNX1 and the mammalian Vps26, Vps29, and Vps35 proteins have been shown to form a large complex in the cytosol, which could be recruited to membranes en bloc (17). There is conflicting biochemical and morphological evidence as to whether SNX2 is a component of the mammalian retromer (8, 14, 15, 17). Whereas yeast two-hybrid analyses showed that SNX2 interacts with SNX1 and, more weakly, with Vps29 and Vps35 (17), coimmunoprecipitation analyses of transiently expressed, epitope-tagged retromer subunits showed an association of SNX1, but not SNX2, with Vps35 (15). In addition, SNX1 and SNX2 were shown to have either similar (8) or different (15) intracellular localizations in cells. There are at least seven other mammalian sorting nexins that also contain PX and BAR domains and that could, in principle, be components of retromers (7). Indeed, SNX15 has been shown to interact with SNX1 (31) as well as with Vps29 and Vps35 (2). Furthermore, SNX1 interacts with various other proteins, including Hrs (10) and enterophilin-1 (32), and there is evidence that both SNX1 and SNX2 bind the cytosolic tails of several signaling receptors to mediate their sorting to lysosomes (15, 16, 23). Moreover, though ubiquitously present, the mRNAs encoding the mammalian Vps26, Vps29, and Vps35 proteins are expressed at disproportionate levels in many tissues (17). Finally, two isoforms of mouse Vps26, termed A and B, were found to localize to endosomes and the plasma membrane, respectively (1, 21, 34).
Like the pattern of protein interactions, the functions ascribed to retromer subunits are also quite diverse. Recent studies involving suppression of retromer subunit expression by RNA interference or the use of retromer-deficient mammalian cells have shown that Vps26 and Vps35 (1, 34) as well as SNX1 (6), but not SNX2 (8), are required for efficient transport of the CI-MPR from endosomes to the trans-Golgi network (TGN). In the absence of any of these subunits, the CI-MPR is diverted to lysosomes, and lysosomal hydrolases become partially missorted (1, 6, 34). The function of the mammalian retromer inferred from these observations is thus analogous to that of the yeast retromer in vacuolar hydrolase sorting. It contrasts, however, with previous proposals that SNX1 and/or SNX2 enhances the targeting of certain signaling receptors to lysosomes, thereby contributing to their down-regulation (12, 15, 23). The latter function has been called into question for SNX1 (6). Recent studies have uncovered yet another function for the mammalian retromer in the regulation of the transcytosis of the polymeric immunoglobulin receptor from the basolateral to the apical plasma membranes of polarized epithelial cells (41). These findings underscore the possibility that mammalian retromer subunits are part of additional complexes that mediate processes other than endosome-to-TGN retrieval. Thus, from all of these data, it is unclear which subunits are unique and obligate components of the mammalian retromer, whether any of these subunits can exist independently of the others (perhaps as components of other complexes), and how they might be involved in diverse cellular processes.
To address some of these uncertainties, we have undertaken a systematic analysis of the assembly, localization, membrane recruitment, and structural and functional requirements of retromer subunits in mammalian cells. Because many of the uncertainties mentioned above arise from the use of transient overexpression of transgenic proteins, our analysis focused on endogenous retromers. Our results indicate that the mammalian retromer consists of two subcomplexes, i.e., a SNX1/2 homodimer or heterodimer and a Vps26-Vps29-Vps35 heterotrimer. Depletion of either SNX1 or SNX2 still allows for the assembly of the remaining SNX2 or SNX1 into a homodimer and has no obvious effect on the levels or subunit assembly of the Vps26-Vps29-Vps35 subcomplex. Depletion of any of the subunits of the latter subcomplex, on the other hand, reduces the levels of the other Vps subunits but has no effect on the levels or assembly of the SNX1/2 dimers. Both subcomplexes therefore behave as independent entities from a biosynthetic standpoint. However, simultaneous depletion of both SNX1 and SNX2 prevents the association of Vps26-Vps29-Vps35 with endosomal membranes; the reciprocal is not the case, as membrane binding of SNX1/2 is unaffected by depletion of the Vps subunits. Finally, depletion of both SNX1 and SNX2 (though not of the individual proteins) results in lysosomal missorting of the CI-MPR similar to that observed upon depletion of either Vps26 or Vps35. Our observations thus shed light on the assembly of endogenous retromers and demonstrate that SNX1 and SNX2 have redundant but essential roles in retromer association with membranes and CI-MPR retrieval.
MATERIALS AND METHODS
Cell culture, cDNA transfections, and RNA interference.
HeLa cells (American Type Culture Collection, Manassas, VA) were maintained in Dulbecco's modified Eagle's medium (Biosource, Rockville, MD) containing 4.5 g/liter glucose, 50 units/ml penicillin, 50 μg/ml of streptomycin, 2 mM l-glutamine, and 10% (vol/vol) fetal bovine serum. For cDNA transfections, cells grown to 80 to 90% confluence in six-well plates were transfected with 4 μg of pcDNA3.1-Vps26-myc, -CFP-SNX1, or -HA-SNX2, using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Cell lysates were prepared 16 h after transfection. For small interfering RNA (siRNA)-mediated suppression of endogenous Vps26, Vps29, Vps35, SNX1, SNX2, or SNX9 (as a negative control), cells grown to 30% confluence were transfected twice, at 24-h intervals, with 80 pmol siRNA oligonucleotide (SMART pool reagent M-013195-00 [Vps26], M-009764-00 [Vps29], M-010894-00 [Vps35], M-017518-00 [SNX1], M-017520-00 [SNX2], or M-017335-00 [SNX9]; Dharmacon, Chicago, IL), using Oligofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Cells were analyzed 48 h after the second round of transfection.
Antibodies.
We used mouse monoclonal antibodies to the following proteins: SNX1 and -2 (BD Biosciences, Franklin Lakes, NJ), α-tubulin (DM1A; Sigma-Aldrich, St. Louis, MO), Lamp-2 (H4A3; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), transferrin receptor (Zymed, San Francisco, CA), CI-MPR (Serotec, Raleigh, NC), and the hemagglutinin (HA) tag (HA.11; Covance, Princeton, NJ). We also used polyclonal antibodies to the following proteins: SNX9 (rabbit antibodies; gift of L. Traub, University of Pittsburgh, Pittsburgh, PA); CI-MPR (rabbit) (20); Vps29, Vps26, Vps35, SNX1, and SNX2 (rabbit) (17); SNX1 (goat antibodies; Santa Cruz Biotechnology Inc., Santa Cruz, CA); and TGN46 (sheep antibodies; Serotec, Raleigh, NC). Goat or donkey antibodies to mouse, rabbit, and goat conjugated to Alexa 488, Alexa 594 or 546, and Alexa 633, respectively, were purchased from Molecular Probes (Eugene, OR). Goat anti-rabbit immunoglobulin G (IgG) conjugated to fluorescein isothiocyanate (FITC) and goat anti-sheep and anti-mouse IgG conjugated to Cy5 were purchased from Jackson ImmunoResearch Laboratories, West Grove, PA, and horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgGs were purchased from Amersham Biosciences, Piscataway, NJ.
Immunoprecipitation.
For coprecipitation analysis of endogenous SNX1 and SNX2, using specific monoclonal antibodies, HeLa cells maintained in 100-mm dishes were rinsed twice with ice-cold phosphate-buffered saline (PBS) and immediately resuspended in 1 ml lysis buffer (0.5% [vol/vol] Triton X-100, 300 mM NaCl, 50 mM Tris-HCl, pH 7.4, 5 mM EDTA) supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN). After 30 min of incubation at 4°C, lysates were centrifuged at 16,000 × g for 15 min. The supernatants were then precleared by incubation for 60 min at 4°C with 30 μl protein G-Sepharose beads (Amersham Pharmacia Biotech, Piscataway, NJ) and centrifugation at 8,000 × g for 5 min. The precleared lysates were subsequently incubated for 2 h at 4°C with 30 μl protein G-Sepharose beads bound to mouse monoclonal antibody to SNX1. Following immunoprecipitation, the beads were washed three times with ice-cold wash buffer (0.1% [wt/vol] Triton X-100, 300 mM NaCl, 50 mM Tris-HCl, pH 7.4) and once with ice-cold PBS. Washed beads were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis with a monoclonal antibody to SNX2.
For immunoprecipitation-recapture analyses of all retromer subunits, using polyclonal antibodies, cells were metabolically labeled for 6 h at 37°C with 0.05 mCi of [35S]methionine-cysteine (Express protein label; Dupont-New England, Boston, MA) per ml of methionine-cysteine-free Dulbecco's modified Eagle's medium supplemented with 10% dialyzed fetal bovine serum. After being labeled, cells were washed twice with ice-cold PBS, and lysates were prepared and precleared as described above. Immunoprecipitations were carried out by incubating the extracts for 2 h at 4°C with polyclonal antibodies to SNX1 or Vps26 bound to 30 μl protein A-Sepharose beads (Amersham Pharmacia Biotech, Piscataway, NJ). Subsequently, beads were washed with wash buffer and PBS as described above. Bound proteins were eluted from the beads by treatment for 5 min at 95°C with 0.1 M Tris-HCl, pH 7.4, 1% (wt/vol) SDS, and 10 mM dithiothreitol. The eluted material was diluted 20-fold with lysis buffer supplemented with 10 mM iodoacetamide, centrifuged at 16,000 × g for 15 min, and then subjected to a second round of immunoprecipitation with protein A-Sepharose beads bound with polyclonal antibodies to SNX1, SNX2, Vps26, Vps29, Vps35, and the β3 subunit of AP-3 as a negative control. After being washed, proteins were subjected to SDS-PAGE, and the 35S-labeled proteins were detected by fluorography.
Yeast two-hybrid assays.
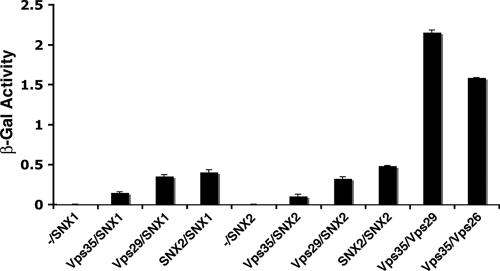
The subcloning of cDNAs encoding full-length human Vps26A in pGADT7 and Vps35 in pGBKT7 has been described before (38). EcoRI-SalI fragments encoding human Vps29 (residues 2 to 183) and human SNX2 were subcloned into the EcoRI-XhoI and EcoRI-SalI sites of the pGADT7 and pGBKT7 vectors (Clontech, Mountain View, CA), respectively. A cDNA encoding human SNX1 was cloned into the EcoRI-XhoI sites of pGADT7. S. cerevisiae strain AH-109 (Clontech) was cotransformed by the lithium acetate procedure with plasmids encoding different SNX and Vps retromer subunits, as indicated in the instructions of a Matchmaker two-hybrid kit (Clontech). The liquid β-galactosidase assay was performed using a commercial kit (Pierce, Woburn, MA). The β-galactosidase activity shown for each prey-bait cotransformation was the result of subtracting the β-galactosidase activity of each prey or bait cotransformed with the empty pGADT7 or pGBKT7 vector from the total β-galactosidase activity.
Preparation of cell lysates, subcellular fractionation, and hydrodynamic analyses.
To prepare total cell lysates, cells from two 100-mm dishes were washed, scraped, and collected in ice-cold Tris-buffered saline (TBS), pH 7.4. Cells were then lysed in 1.0 ml of TBS containing 0.5% (wt/vol) Triton X-100 and protease inhibitors by 20 passages through a 25-gauge needle, incubated on ice for 30 min, and then centrifuged at 16,000 × g for 15 min. For subcellular fractionation, cells were homogenized in 1.0 ml TBS-protease inhibitors in the absence of detergent by 20 passages through a 25-gauge needle and then centrifuged at 1,500 × g for 15 min to obtain a postnuclear supernatant fraction. This fraction was subsequently subjected to ultracentrifugation for an additional hour at 125,000 × g to generate cytosolic and membrane fractions.
For gel filtration experiments, both whole-cell lysates and cytosol were first passed through a 0.45-μm filter unit (Millipore, Bedford, MA), and 300 μl of filtered sample was loaded onto a Superdex 200 HR column (Amersham Pharmacia Biotech), equilibrated, and eluted at 4°C with TBS, pH 7.4. Fractions were collected and analyzed by immunoblotting. For sucrose gradient fractionation, 300 μl of whole-cell lysate was layered on top of a linear 2 to 10% (wt/vol) sucrose gradient (total volume, 12 ml) in TBS, pH 7.4. The samples were centrifuged in an SW-41 rotor (Beckman Instruments, Fullerton, CA) at 39,000 rpm for 16 h at 4°C. Fractions were collected from the bottom of the tube and analyzed by immunoblotting. Hydrodynamic parameters were calculated as described previously (26).
Immunofluorescent staining and confocal microscopy.
Cells grown on coverslips were rinsed with PBS and fixed for 15 min with 4% (wt/vol) paraformaldehyde in PBS at room temperature. Cells were rinsed twice more with PBS, and excess paraformaldehyde was quenched with PBS containing 20 mM glycine, pH 8.0, and 75 mM NH4Cl for 15 min at room temperature. The cells were again washed with PBS and permeabilized with 0.025% (wt/vol) saponin in blocking solution (PBS containing 5% [vol/vol] goat serum and 7 mg/ml fish skin gelatin) for 10 min at 37°C in a humidified chamber. Cells were immunostained with primary antibodies for 2 h at 37°C, followed by incubation with fluorescently labeled secondary antibodies for 1 h.
In experiments in which cells were treated with wortmannin, cells were washed once and incubated in minimum essential Eagle medium (MEM; Sigma-Aldrich) containing 2.5 g/liter NaHCO3, 20 mM HEPES, pH 7.4, and 0.6% (wt/vol) bovine serum albumin (MEM-BSA) at 37°C for 30 min. Cells were then incubated with dimethyl sulfoxide or 200 nM wortmannin in dimethyl sulfoxide for either 5, 10, or 20 min and then immediately fixed and immunostained with rabbit anti-Vps26 and mouse anti-SNX1 and anti-SNX2, followed by the appropriate secondary antibodies, as described above.
For CI-MPR antibody uptake assays, HeLa cells were rinsed twice with MEM-BSA and then incubated with a mouse monoclonal antibody to the luminal domain of the CI-MPR (described above) in MEM-BSA for 2 h at 37°C. After this incubation, the cells were rinsed, fixed, and immunostained with goat anti-SNX1 and rabbit anti-SNX2 antibodies, followed by the appropriate secondary antibodies, as described above.
Imaging was performed on a TCS SP-2 confocal microscope (Leica, Deerfield, IL) equipped with argon-krypton, 543/594-nm helium-neon, and 633-nm helium-neon lasers. Images were acquired using a ×63 Plan-Apochromat oil objective (numerical aperture, 1.4) and the appropriate filter combination. Settings were as follows: photomultipliers were set to 500 to 700 V, the pinhole was set at 1 airy, the zoom setting was 3.0 to 4.0, and a Kalman filter (n = 8) was used. The images (512 by 512 pixels) were saved as TIFF files, contrast was adjusted with Photoshop (Adobe, San Jose, CA), and images were imported into Freehand MX (Macromedia, San Francisco, CA).
RESULTS
Association of human retromer subunits into two independently assembling subcomplexes.
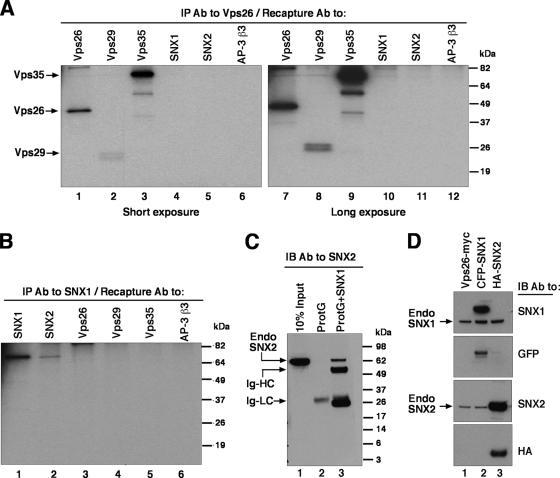
To examine the assembly of endogenous retromer subunits, we performed immunoprecipitation-recapture analyses (3) of Triton X-100 extracts of [35S]methionine-cysteine-labeled human HeLa cells (Fig. 2). This procedure consisted of an initial immunoprecipitation with a polyclonal antibody to one of the retromer subunits, followed by denaturation of the complex in SDS and recapture with polyclonal antibodies to each of the five retromer subunits. Immunoprecipitation with a polyclonal antibody to Vps26 resulted in the recapture of Vps26 together with Vps29 and Vps35, but not SNX1 and SNX2 (Fig. 2A). Conversely, immunoprecipitation with a polyclonal antibody to SNX1 resulted in the recapture of SNX1 together with SNX2, but not Vps26, Vps29, and Vps35 (Fig. 2B). To rule out that the recapture of SNX1 with SNX2 was due to cross-reactivity, we performed another experiment in which a Triton X-100 extract from unlabeled HeLa cells was subjected to immunoprecipitation with a monoclonal antibody to SNX1, and the immunoprecipitate was subsequently analyzed by immunoblotting with a monoclonal antibody to SNX2 instead of the polyclonal antibodies to SNX1 and SNX2 used in the previous experiment. This confirmed the presence of SNX2 in the SNX1 immunoprecipitates (Fig. 2C, lane 3). Control experiments showed that both monoclonal antibodies were specific for their corresponding antigens (Fig. 2D). From these results, we concluded that, at least in Triton X-100 extracts, the five putative subunits of endogenous human retromer occur as components of two separate subcomplexes, with one comprising Vps26, Vps29, and Vps35 and the other comprising SNX1 and SNX2.
FIG. 2.
Coimmunoprecipitation of endogenous retromer subunits. (A and B) HeLa cells metabolically labeled with [35S]methionine-cysteine were lysed with 0.5% (vol/vol) Triton X-100 and immunoprecipitated with rabbit anti-Vps26 (A) or rabbit anti-SNX1 (B). Washed immunoprecipitates were eluted by heating at 95°C for 5 min in the presence of 10 mM dithiothreitol and 1% (wt/vol) SDS and later diluted with lysis buffer. Samples were subjected to recapture with rabbit antibodies to Vps26, Vps29, Vps35, SNX1, SNX2, and the β3 subunit of the AP-3 complex as a negative control. Washed immunoprecipitates were analyzed by 12% acrylamide SDS-PAGE followed by fluorography. Two different exposure times are shown in panel A to better document the signal corresponding to Vps29 (this protein migrates as a doublet by SDS-PAGE). (C) HeLa cells were lysed with 0.5% (vol/vol) Triton X-100 and immunoprecipitated with (lane 3) or without (lane 2) a monoclonal antibody to SNX1. After being washed, the immunoprecipitates were eluted in sample buffer and analyzed by 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting with a monoclonal antibody to SNX2. Ten percent of the input lysate was loaded in lane 1 as a control. (D) HeLa cells transfected with plasmids encoding Vps26-Myc (lane 1), CFP-SNX1 (lane 2), and HA-SNX2 (lane 3) were lysed and subjected to SDS-PAGE and immunoblotting with monoclonal antibodies to SNX1, SNX2, and HA and a polyclonal antibody to green fluorescent protein (GFP). The positions of molecular mass markers (in kDa) are indicated on the right in panels A to C. Abbreviations: IP, immunoprecipitation; Ab, antibody; IB, immunoblotting; Endo, endogenous; Ig-HC; immunoglobulin heavy chain; Ig-LC, immunoglobulin light chain.
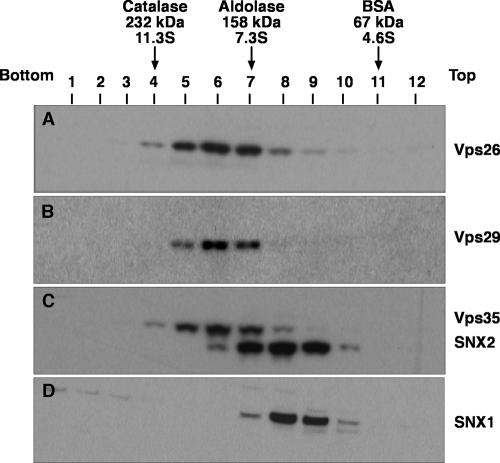
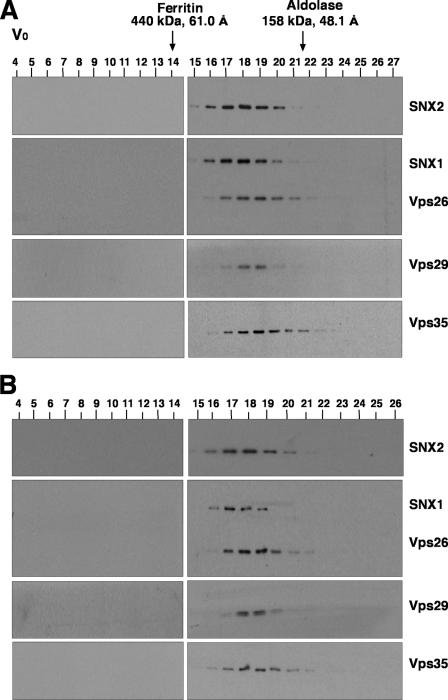
To further characterize the properties of the retromer subunits in Triton X-100 extracts, we performed sedimentation velocity analyses on linear sucrose gradients. In agreement with the coprecipitation analyses described above, this technique showed that Vps26, Vps29, and Vps35 cosedimented with a coefficient of 8.1 S, whereas SNX1 and SNX2 cosedimented with a coefficient of 6.1 S (Fig. 3). Similarly, gel filtration analyses of both a Triton X-100 extract (Fig. 4A) and a cytosolic extract (Fig. 4B) from HeLa cells showed that Vps26, Vps29, and Vps35 coeluted, with a peak in fractions 18 and 19 (corresponding to a Stokes radius of 56 Å), whereas SNX1 and SNX2 coeluted, with a peak in fractions 17 and 18 (corresponding to a Stokes radius of 58 Å). Although this difference in the elution behaviors of the two subcomplexes was small, it could be observed on the same blots (for example, see the blots simultaneously probed for both SNX1 and Vps26 in Fig. 4) and was highly reproducible from experiment to experiment. Thus, Vps26-Vps29-Vps35 and SNX1/2 behaved as two separate subcomplexes, irrespective of whether they were derived from Triton X-100 extracts of whole cells or from cytosolic extracts. The fact that SNX1/2 appeared “smaller” than Vps26-Vps29-Vps35 by sedimentation velocity but slightly “larger” than Vps26-Vps29-Vps35 by gel filtration indicates that SNX1/2 has a more asymmetric (i.e., extended) shape. Indeed, from the sedimentation velocity and gel filtration parameters, we calculated (26) that the Vps26-Vps29-Vps35 and SNX1/2 subcomplexes have frictional ratios of 2.1 and 2.7, respectively.
FIG. 3.
Sedimentation velocity analysis of retromer subunits. Triton X-100 extracts of HeLa cells were fractionated by centrifugation in linear 2 to 10% (wt/vol) sucrose gradients as described in Materials and Methods. Samples representing 4% of the volume of each fraction were analyzed by 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting with antibodies to Vps26 (A), Vps29 (B), and SNX1 (D) or jointly with antibodies to Vps35 and SNX2 (C). The positions in the gradient, molecular masses, and sedimentation coefficients of protein standards are indicated at the top.
FIG. 4.
Gel filtration analysis of retromer subunits. Triton X-100 extracts (A) and cytosol (B) from HeLa cells, prepared as indicated in Materials and Methods, were subjected to gel filtration on a calibrated Superdex 200 HR column. Samples representing 2% of the volume of each fraction were analyzed by 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting with antibodies to each of the retromer subunits. The void volume (V0) and the positions, molecular masses, and Stokes radii of standard proteins are indicated at the top.
Biosynthetic relationships of retromer subunits confirm their assembly into two subcomplexes.
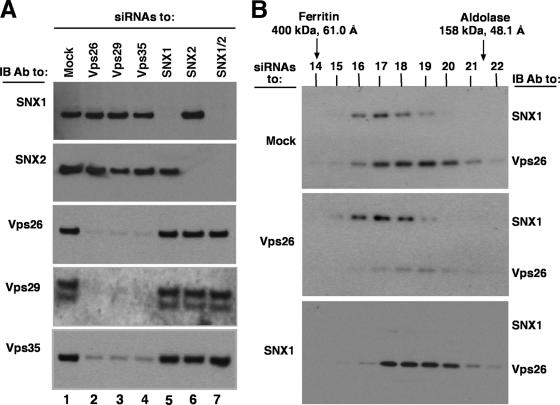
To further analyze the relationships among the retromer subunits, we examined the effects of depleting cells of one subunit on the levels and hydrodynamic behavior of the other subunits. This was done by treating HeLa cells with siRNAs directed to each retromer subunit and then analyzing cell extracts by immunoblotting or gel filtration (Fig. 5). We observed that depletion of Vps26, Vps29, or Vps35 resulted in reduced levels of not only the target of the siRNA but also the other subunits of this subcomplex. In contrast, depletion of any of these subunits did not affect the levels of SNX1 and SNX2 (Fig. 5A). Together with previous data (1, 34), this indicated that Vps26, Vps29, and Vps35 are destabilized in the absence of any one of their subcomplex partners, as is the case for other unassembled subunits of multiprotein complexes (22). In contrast, depletion of SNX1, SNX2, or both only affected the subunits that were directly targeted by the siRNAs, not the other subunits (Fig. 5A). These observations demonstrated the biosynthetic interdependence of Vps26, Vps29, and Vps35 and their independence from SNX1 and SNX2, supporting the notion that both subcomplexes behave as separate entities from a biosynthetic standpoint. The fact that depletion of SNX1 did not affect the levels of SNX2, and vice versa, might be explained by the ability of these proteins to assemble into homodimers in addition to heterodimers (16, 18, 24). We also examined the effect of depleting Vps26 or SNX1 on the elution of each subcomplex in gel filtration. We found that depletion of Vps26 did not alter the behavior of SNX1, and vice versa (Fig. 5B). This confirmed that the sizes estimated by gel filtration of normal cell extracts correspond to those of the subcomplexes (despite the proximity of their peaks) and that each subcomplex behaves independently of the other in solution.
FIG. 5.
Effects of depleting retromer subunits on the levels and gel filtration behaviors of the other subunits. (A) Triton X-100 extracts from mock-treated cells or cells treated with siRNA to Vps26, Vps29, Vps35, SNX1, SNX2, or SNX1/SNX2 together were subjected to 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting (IB) with antibodies to the indicated proteins. Equal amounts of total protein, as quantified by the bicinchoninic acid assay, were loaded in each lane. (B) Triton X-100 extracts from mock-treated cells or cells treated with siRNA to Vps26 or SNX1 were subjected to gel filtration on a calibrated Superdex 200 HR column. Five percent of the volume of each fraction was analyzed by 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting with a mixture of antibodies to Vps26 and SNX1. The positions, molecular masses, and Stokes radii of standard proteins are indicated at the top. Notice that siRNA depletion of Vps26 did not alter the elution profile of SNX1 (or SNX2 [data not shown]). Similarly, siRNA depletion of SNX1 did not affect the elution properties of Vps26 (or Vps29 and Vps35 [data not shown]).
Interactions of the SNX and Vps subunits of retromer detected by a yeast two-hybrid system.
In the experiments described so far, we failed to detect any physical or biosynthetic interaction between the SNX and Vps subunits of the mammalian retromer. Likewise, pull-down experiments performed by us (data not shown) and others (11) did not show any association between components of the two subcomplexes. These negative results indicate that interactions between the SNX and Vps subunits of retromer are of relatively low affinity. We thus turned to the yeast two-hybrid system, which is particularly suited to detecting weak interactions. Analyses using the LexA system gave unacceptable levels of self-activation for the SNX subunits. On the other hand, the Gal4 system, in particular with the use of a liquid β-galactosidase assay, gave much more reliable results. Using this system, we found that both SNX1 and SNX2 interacted with Vps29 and Vps35, albeit with lower avidities than those for interactions between Vps29 and Vps35 (Fig. 6). In contrast, we did not detect interactions between SNX1 or SNX2 and Vps26 (data not shown). We also detected interactions between SNX1 and SNX2, consistent with the ability of these proteins to form heterodimers (Fig. 6).
FIG. 6.
Yeast two-hybrid analysis of interactions between the SNX and Vps subunits of the mammalian retromer. S. cerevisiae strain AH109 was cotransformed with the combinations of SNX and Vps subunits of retromer indicated in the figure. For each pair, the first protein was expressed as a fusion with the Gal4 DNA-binding domain from the pGBKT7 plasmid, while the second protein was expressed as a fusion with the Gal4 transcription activation domain from the pGADT7 plasmid. Minus signs indicate the absence of any retromer subunit in pGBKT7. Interactions were quantified using a liquid β-galactosidase (β-Gal) assay. Activity values (in arbitrary units) are the means ± standard deviations for triplicate determinations. The experiment shown in the figure is representative of four experiments that gave similar results.
Requirement of SNX1 or SNX2 for Vps26-Vps29-Vps35 association with endosomes.
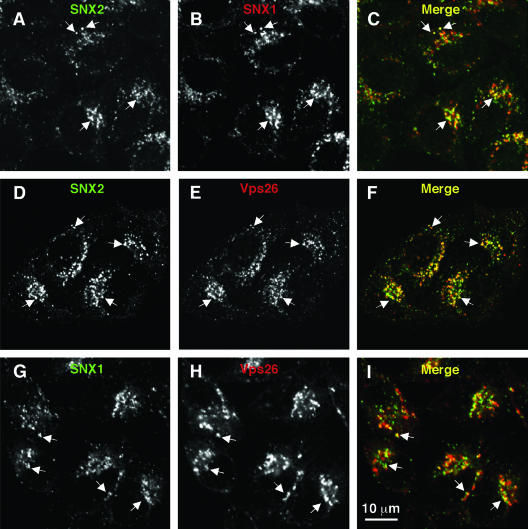
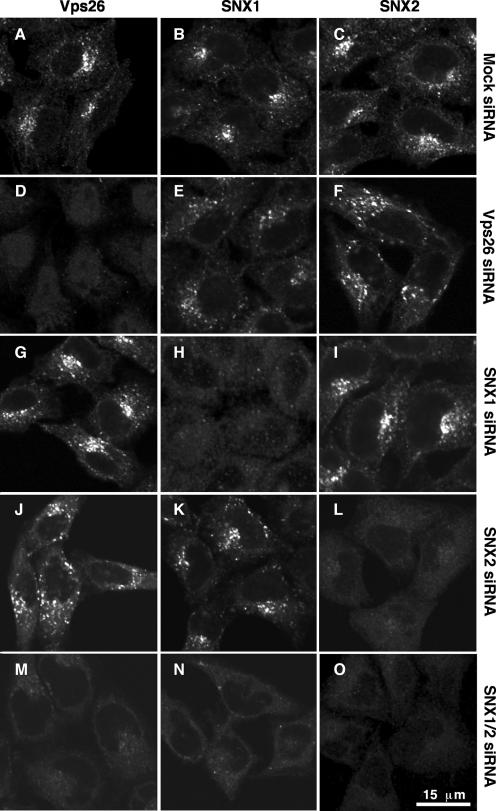
Consistent with the interactions detected using the yeast two-hybrid system, SNX1, SNX2, and Vps26 colocalized to the same punctate cytoplasmic foci by immunofluorescence microscopy of HeLa cells (Fig. 7A to I). Previous studies showed that these foci correspond to endosomes (1, 6, 8, 15, 24, 34, 40, 43, 44). In addition, some cells exhibited colocalization of SNX1, SNX2, and Vps26 to tubules (data not shown) that likely correspond to the retromer-decorated endosomal tubules previously observed by immunoelectron microscopy (1, 6, 8). The ability to suppress expression of individual retromer subunits by using siRNAs allowed us to assess the subunit requirements for association of both subcomplexes with endosomes. Immunofluorescence microscopy of mock-treated HeLa cells showed similar staining patterns for endogenous Vps26, SNX1, and SNX2 (Fig. 8A to C). Depletion of Vps26 caused a drastic reduction in staining for Vps26 but had no effect on SNX1 and SNX2 staining (Fig. 8D to F). Treatment with siRNA for SNX1 or SNX2 abolished staining for the target SNX but did not affect staining for the other SNX or for Vps26 (Fig. 8G to L). However, combined depletion of SNX1 and SNX2 resulted in a reduction in the punctate staining for Vps26 (Fig. 8M to O). Since depletion of SNX1 and SNX2 did not alter the total level of Vps26 protein (Fig. 5A) and since EEA1 remained associated with endosomes (see Fig. S1 in the supplemental material), the reduction in Vps26 staining is attributable to its dissociation from membranes. From these observations, we concluded that SNX1 and SNX2 can be recruited to endosomes independently of one another and of the Vps26-Vps29-Vps35 subcomplex. In contrast, efficient endosomal association of the Vps26-Vps29-Vps35 subcomplex requires the presence of either SNX1 or SNX2. Treatment of live cells with 200 nM of the phosphatidylinositol 3-kinase inhibitor wortmannin caused dissociation of SNX1, SNX2, and Vps26, with similar kinetics (see Fig. S2 in the supplemental material), consistent with a requirement of the phosphatidylinositol 3-phosphate-binding activity of SNX1 and SNX2 (8, 12, 43) for Vps26-Vps29-Vps35 association with membranes.
FIG. 7.
Colocalization of endogenous SNX1, SNX2, and Vps26 in HeLa cells. The intracellular localization of SNX2 (A and D), SNX1 (B and G), and Vps26 (E and H) was assessed in fixed and permeabilized cells by indirect immunofluorescence and confocal microscopy. (A, D, and G) Alexa 488, green channel. (B, E, and H) Alexa 546, red channel. (C, F, and I) Merged images. Yellow indicates colocalization. Examples of foci where proteins colocalize are indicated by arrows.
FIG. 8.
Requirement of SNX1 or SNX2 for association of the Vps26-Vps29-Vps35 subcomplex with endosomal membranes. HeLa cells were treated twice, at 24-h intervals, with an inactive siRNA to Vps35 (mock; A to C) or active siRNA to Vps26 (D to F), SNX1 (G to I), SNX2 (J to L), or SNX1 and SNX2 together (M to O). At 48 h posttreatment, the cellular distributions of Vps26 (A, D, G, J, and M), SNX1 (B, E, H, K, and N), and SNX2 (C, F, I, L, and O) were assessed by indirect immunofluorescence staining using mouse monoclonal antibodies to the corresponding SNX proteins and a rabbit polyclonal antibody to Vps26, followed by FITC-conjugated goat anti-rabbit IgG and Cy5-conjugated goat anti-mouse IgG. Images were captured by confocal microscopy. The same confocal microscope settings were used for imaging of all mock- and siRNA-treated cells.
Functional redundancy of SNX1 and SNX2 in the sorting of mannose 6-phosphate receptors.
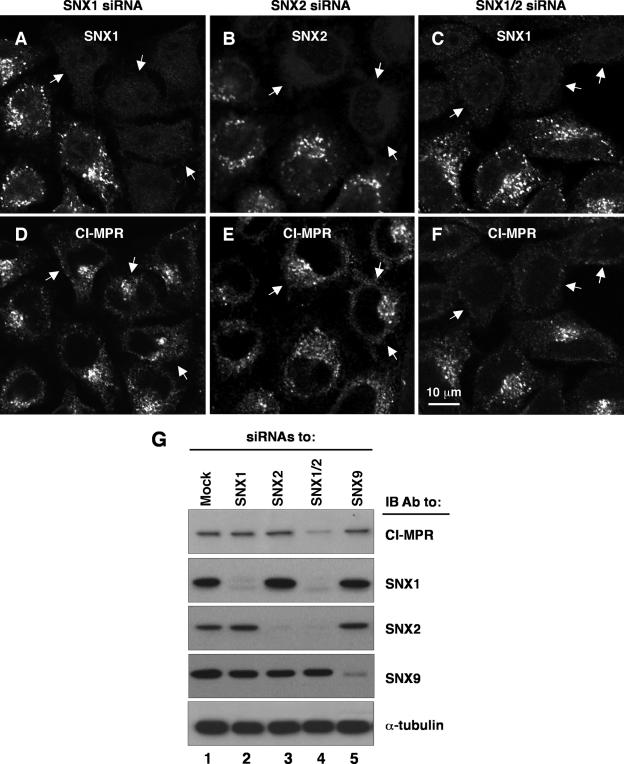
Previous studies showed that depletion of Vps26 or Vps35 causes missorting of the CI-MPR to lysosomes, which is manifested by decreased staining for the receptor by immunofluorescence microscopy and decreased levels of receptor protein by immunoblotting (1, 34). We used similar assays to examine the effect of depleting SNX1, SNX2, or both on endogenous CI-MPR. Although depletion of SNX1 and/or SNX2 by using siRNAs was generally very efficient (>90%, as measured by immunoblotting) (Fig. 5A), we found a few islands of cells that still expressed the target SNX (Fig. 9A to C). We took advantage of this local inhomogeneity of the cell population to assess the status of the CI-MPR in SNX1/2-positive and -negative cells in the same field of view. We observed that single depletion of SNX1 or SNX2 had little or no effect on the distribution or staining intensity of the CI-MPR (Fig. 9D and E). However, combined depletion of SNX1 and SNX2 caused a dramatic decrease in staining for the CI-MPR, with the residual staining being located on structures that appeared more disperse (Fig. 9F). The distributions of other organellar markers, such as the transferrin receptor and Lamp-2 (data not shown), were not affected in the SNX1/2-depleted cells, indicating that the effects on the CI-MPR were specific. Immunoblot analysis also showed that single depletion of SNX1 or SNX2 did not affect CI-MPR levels but that combined depletion of both SNXproteins greatly decreased those levels (Fig. 9G). As a control, we showed that depletion of SNX9 had no effect on CI-MPR levels (Fig. 9G). Up to 10% of the CI-MPR is normally expressed at the cell surface, from where it can be internalized and transported to the TGN (9). We observed that whereas mock-treated (control) cells or cells depleted of SNX1 or SNX2 took up antibody to CI-MPR and delivered it to the juxtanuclear area (see Fig. S3A to L in the supplemental material), cells depleted of both SNX1 and SNX2 showed greatly reduced uptake and more disperse localization of the antibody (see Fig. S3M to P in the supplemental material). This is consistent with the decreased levels of CI-MPR (Fig. 9) and additionally indicates that transport of internalized antibody to the TGN is impaired in SNX1/2-depleted cells. From these experiments, we concluded that SNX1 and SNX2 play redundant roles in the sorting of the CI-MPR, which agrees with the redundancy of their requirements for association of the Vps26-Vps29-Vps35 subcomplex with endosomes (Fig. 8).
FIG. 9.
Functional redundancy of SNX1 and SNX2 in sorting of the CI-MPR. (A to F) HeLa cells treated with siRNAs to SNX1 (A and D), SNX2 (B and E), and SNX1 and SNX2 together (C and F) were analyzed by immunofluorescence staining and confocal microscopy. Cells were double stained with rabbit polyclonal antibodies to the corresponding SNX proteins (A to C) and a mouse monoclonal antibody to the CI-MPR (D to F), followed by FITC-conjugated goat anti-rabbit IgG and Cy5-conjugated goat anti-mouse IgG. As internal controls, a minority of cells that did not respond to the siRNA treatment were imaged next to the majority of cells that responded. Arrows point to cells depleted of the indicated SNX protein. (G) Extracts of HeLa cells treated with siRNAs to the proteins indicated at the top were analyzed by 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting (IB) with antibodies to the proteins indicated on the right. Equal amounts of total protein were loaded. Blots were also probed with antibody to α-tubulin as a loading control.
DISCUSSION
The mammalian retromer, like its yeast counterpart, participates in the retrieval of acid hydrolase receptors from endosomes to the TGN (1, 6, 34). This function is analogous to that of protein coats that function in vesicle formation and cargo sorting at various stages of the endocytic and secretory pathways (4). A recent study has identified a “fuzzy” electron-dense layer on plant vesicle fractions enriched in Vps35, although it has not been demonstrated that this deposit corresponds to retromer (30). The characterization of the quaternary structure and functional roles of the different subunits of the mammalian retromer has lagged behind that of protein coats in large part due to the limitations inherent in the use of overexpressed transgenic proteins in previous studies. This prompted us to undertake a detailed analysis of the physical and functional relationships of the endogenous retromer complex in human cells.
Human retromer consists of SNX1/2 and Vps26-Vps29-Vps35 subcomplexes.
Our results show that five putative subunits of the endogenous mammalian retromer occur as components of two independently assembling subcomplexes, SNX1/2 and Vps26-Vps29-Vps35, in both cytosolic and detergent extracts of whole cells. The existence of a stable Vps26-Vps29-Vps35 subcomplex was previously inferred from the association of the recombinant subunits (11). These findings are at variance with the previous proposal that the mammalian retromer exists as a stable five-subunit complex that is recruited from the cytosol to membranes en bloc (17). The two retromer subcomplexes exhibit different biosynthetic relationships among their constituent subunits. SNX1 and SNX2 behave as alternate heterodimers or homodimers. Depletion of one of these proteins does not affect the stability of the other, because the remaining protein can form homodimers. Vps26-Vps29-Vps35, on the other hand, behaves as an obligate heterotrimer, for which depletion of any one subunit results in disappearance of the others. This interdependence of Vps26, Vps29, and Vps35 makes it unlikely that these subunits can exist separately or as components of other complexes, as previously speculated. Diversification of the retromer, however, is possible through the assembly of different subunit isoforms (21).
SNX1/2 homodimers or heterodimers are required for association of the Vps26-Vps29-Vps35 subcomplex with endosomes.
The ability to deplete cells of different retromer subunits by using siRNAs allowed us to examine the structural requirements for the associations of both subcomplexes with membranes. The SNX1/2 subcomplex was found to associate with membranes independently of the Vps26-Vps29-Vps35 subcomplex. The converse was not the case, as association of the Vps26-Vps29-Vps35 subcomplex with membranes required the presence of either SNX1 or SNX2. This behavior of mammalian Vps26-Vps29-Vps35 appears distinct from that of yeast Vps26p, which remains associated with membranes in deletion mutants lacking the SNX-like subunit Vps5p or Vps17p (35). Together with previous findings, these observations suggest that SNX1/2 heterodimers or homodimers are recruited from the cytosol to membranes by binding of their PX domains to phosphatidylinositol 3-phosphate or other phosphoinositides and of their BAR domains to highly curved membranes (6, 8, 39), through what has been referred to as “coincidence sensing” (6). This recruitment could be regulated by an ortholog of the yeast phosphatidylinositol 3-kinase, Vps34p (5), which localizes to endosomes in mammalian cells (39). The extended N-terminal segments of SNX1/2 would then bind cytosolic Vps26-Vps29-Vps35 (demonstrated for SNX1 in reference 15), resulting in the association of this subcomplex with membranes. The interaction between the two subcomplexes must be of low affinity or transient because it could not observed in cytosolic extracts and did not withstand solubilization of cells with Triton X-100. In addition, it could not be demonstrated by pull-down assays (11; our unpublished observations) but could be shown only by yeast two-hybrid assays (17; this study). This suggests that additional interactions might contribute to the stabilization of Vps26-Vps29-Vps35 on membranes. One such interaction could be the binding of the cytosolic tails of cargo proteins to the corresponding cargo recognition subunits. A recent study has also shown an interaction of the GTP-bound form of Rab7 with the Vps26-Vps29-Vps35 subcomplex of Entamoeba histolytica (27). It is therefore likely that binding to SNX1 and SNX2 is just one of several interactions that cooperate to recruit the Vps26-Vps29-Vps35 complex to membranes. In any event, our results indicate that in addition to promoting membrane remodeling and tubulation (6, 8), SNX1 and SNX2 enable association of the cargo recognition Vps26-Vps29-Vps35 subcomplex with membranes.
SNX1 and SNX2 play redundant but essential roles in CI-MPR trafficking.
We also found that the presence of either SNX1 or SNX2 was required for proper sorting of the CI-MPR. Depletion of one of these proteins had no detectable effect, but depletion of both resulted in decreased levels and dispersal of the residual CI-MPR, a phenotype that is similar to that caused by depletion of Vps26 or Vps35 (1, 34). This is consistent with the requirement of either SNX1 or SNX2 for the association of Vps26-Vps29-Vps35 with membranes. The reduced level and dispersal of the CI-MPR most likely arise from the inability of the receptor to return from endosomes to the TGN and its consequent missorting to lysosomes, as previously shown for Vps26-depleted cells (1, 34). Carlton et al. (6) showed that depletion of SNX1 alone was sufficient to elicit these effects on CI-MPR, but we found that both SNX1 and SNX2 need to be depleted to observe a clear effect. Carlton et al. (8) also found little observable effect on the steady-state localization of the CI-MPR on SNX2 depletion, although they did find a subtle effect on CI-MPR trafficking in kinetic uptake assays. We do not know the reason for these differences with our findings, but one possible explanation could be the abundance of SNX2 relative to SNX1 in the strains of HeLa cells used by the two groups (HeLaM cells were used for references 6 and 8, and HeLa cells from the ATCC were used in our study).
Concluding remarks.
By several criteria, SNX1 and SNX2 behave as interchangeable subunits of the same subcomplex. As far as the retromer is concerned, they essentially behave as variant isoforms of the same protein, which is consistent with their 63% identity at the amino acid level. First, they coassemble, although the absence of one does not affect the stability of the other. Second, they largely colocalize on endosomes and derived tubules. Finally, either SNX1 or SNX2 is sufficient to allow association of Vps26-Vps29-Vps35 with membranes and proper sorting of the CI-MPR. Clearly, other SNX proteins cannot compensate for the loss of SNX1 and SNX2. We cannot rule out, however, that SNX1 and SNX2 are redundant, obligatory components of an as yet unidentified heterodimer with another SNX protein. Since the depletion of Vps26-Vps29-Vps35 has no effect on the association of SNX1/2 with membranes, our results also do not exclude that SNX1 and SNX2 could perform additional functions unrelated to the retromer, as previously shown (12, 15, 23). Our observations are in line with results from gene ablation studies with the mouse. These studies showed that mice lacking SNX1 or SNX2 were viable, whereas mice lacking both proteins died at midgestation (14, 33), a phenotype similar to that caused by ablation of the Vps26 gene (also known as Hβ58) (25). This redundant relationship of the SNX1/2 subunits is distinct from that of the two SNX-like subunits of the yeast retromer, i.e., Vps5p (the ortholog of both SNX1 and SNX2) and Vps17p, both of which are essential for function (19). Hence, yeast Vps5p-Vps17p has the characteristics of an obligate heterodimer. Since Vps17p orthologs can only be found in fungi (R. Rojas and J. S. Bonifacino, unpublished observations), it is likely that the mode of interaction and functional requirements of the SNX subunits of the mammalian retromer reported here will be more generally applicable to other eukaryotic organisms.
Supplementary Material
Acknowledgments
We thank X. Zhu and H.-T. Tsai for expert technical assistance, L. Traub for his kind gift of antibody to SNX9, and R. Mattera and P. McCormick for critical reviews of the manuscript. We also thank Cecilia Arighi for her technical advice and helpful discussions.
This work was funded by the intramural program of the National Institute of Child Health and Human Development.
Footnotes
Published ahead of print on 13 November 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Arighi, C. N., L. M. Hartnell, R. C. Aguilar, C. R. Haft, and J. S. Bonifacino. 2004. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr, V. A., S. A. Phillips, S. I. Taylor, and C. R. Haft. 2000. Overexpression of a novel sorting nexin, SNX15, affects endosome morphology and protein trafficking. Traffic 1:904-916. [DOI] [PubMed] [Google Scholar]
- 3.Bonifacino, J. S., and E. C. Dell'Angelica. 1998. Immunoprecipitation, p. 7.2.1-7.2.21. In J. S. Bonifacino, M. Dasso, J. B. Harford, J. Lippincott-Schwartz, and K. Yamada (ed.), Current protocols in cell biology. John Wiley & Sons, New York, NY.
- 4.Bonifacino, J. S., and B. S. Glick. 2004. The mechanisms of vesicle budding and fusion. Cell 116:153-166. [DOI] [PubMed] [Google Scholar]
- 4a.Bonifacino, J. S., and R. Rojas. 2006. Retrograde transport from endosomes to the trans-Golgi network. Rev. Mol. Cell Biol. 7:568-579. [DOI] [PubMed] [Google Scholar]
- 5.Burda, P., S. M. Padilla, S. Sarkar, and S. D. Emr. 2002. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. J. Cell Sci. 115:3889-3900. [DOI] [PubMed] [Google Scholar]
- 6.Carlton, J., M. Bujny, B. J. Peter, V. M. Oorschot, A. Rutherford, H. Mellor, J. Klumperman, H. T. McMahon, and P. J. Cullen. 2004. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr. Biol. 14:1791-1800. [DOI] [PubMed] [Google Scholar]
- 7.Carlton, J., M. Bujny, A. Rutherford, and P. Cullen. 2005. Sorting nexins—unifying trends and new perspectives. Traffic 6:75-82. [DOI] [PubMed] [Google Scholar]
- 8.Carlton, J. G., M. V. Bujny, B. J. Peter, V. M. Oorschot, A. Rutherford, R. S. Arkell, J. Klumperman, H. T. McMahon, and P. J. Cullen. 2005. Sorting nexin-2 is associated with tubular elements of the early endosome, but is not essential for retromer-mediated endosome-to-TGN transport. J. Cell Sci. 118:4527-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, H. J., J. Yuan, and P. Lobel. 1997. Systematic analysis of the cation-independent mannose 6-phosphate receptor/insulin-like growth II factor receptor cytoplasmic domain. J. Biol. Chem. 272:7003-7012. [DOI] [PubMed] [Google Scholar]
- 10.Chin, L. S., M. C. Raynor, X. Wei, H. Q. Chen, and L. Li. 2001. Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J. Biol. Chem. 276:7069-7078. [DOI] [PubMed] [Google Scholar]
- 11.Collins, B. M., C. F. Skinner, P. J. Watson, M. N. Seaman, and D. J. Owen. 2005. Vps29 has a phosphoesterase fold that acts as a protein interaction scaffold for retromer assembly. Nat. Struct. Mol. Biol. 12:594-602. [DOI] [PubMed] [Google Scholar]
- 12.Cozier, G. E., J. Carlton, A. H. McGregor, P. A. Gleeson, R. D. Teasdale, H. Mellor, and P. J. Cullen. 2002. The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J. Biol. Chem. 277:48730-48736. [DOI] [PubMed] [Google Scholar]
- 13.Damen, E., E. Krieger, J. Nielsen, J. Eygensteyn, and J. V. Leeuwen. 2006. The human Vps29 retromer component is a metallo-phosphoesterase for a cation-independent mannose 6-phosphate receptor substrate peptide. Biochem. J. 398:399-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin, C. T., J. Trejo, and T. Magnuson. 2005. Genetic evidence for a mammalian retromer complex containing sorting nexins 1 and 2. Proc. Natl. Acad. Sci. USA 102:15173-15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gullapalli, A., T. A. Garrett, M. M. Paing, C. T. Griffin, Y. Yang, and J. Trejo. 2004. A role for sorting nexin 2 in epidermal growth factor receptor down-regulation: evidence for distinct functions of sorting nexin 1 and 2 in protein trafficking. Mol. Biol. Cell 15:2143-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haft, C. R., M. de la Luz Sierra, V. A. Barr, D. H. Haft, and S. I. Taylor. 1998. Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol. Cell. Biol. 18:7278-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haft, C. R., M. de la Luz Sierra, R. Bafford, M. A. Lesniak, V. A. Barr, and S. I. Taylor. 2000. Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: assembly into multimeric complexes. Mol. Biol. Cell 11:4105-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haft, C. R., L. Sierra, V. A. Barr, R. Bafford, and S. I. Taylor. 1999. Sorting nexins (SNX) 1 and 2: interaction domains involved in self association and associations with human retromer proteins. Mol. Biol. Cell 10:114a. [Google Scholar]
- 19.Horazdovsky, B. F., B. A. Davies, M. N. Seaman, S. A. McLaughlin, S. Yoon, and S. D. Emr. 1997. A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol. Biol. Cell 8:1529-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kametaka, S., R. Mattera, and J. S. Bonifacino. 2005. Epidermal growth factor-dependent phosphorylation of the GGA3 adaptor protein regulates its recruitment to membranes. Mol. Cell. Biol. 25:7988-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerr, M. C., J. S. Bennetts, F. Simpson, E. C. Thomas, C. Flegg, P. A. Gleeson, C. Wicking, and R. D. Teasdale. 2005. A novel mammalian retromer component, Vps26B. Traffic 6:991-1001. [DOI] [PubMed] [Google Scholar]
- 22.Klausner, R. D., J. Lippincott-Schwartz, and J. S. Bonifacino. 1990. The T cell antigen receptor: insights into organelle biology. Annu. Rev. Cell Biol. 6:403-431. [DOI] [PubMed] [Google Scholar]
- 23.Kurten, R. C., D. L. Cadena, and G. N. Gill. 1996. Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science 272:1008-1010. [DOI] [PubMed] [Google Scholar]
- 24.Kurten, R. C., A. D. Eddington, P. Chowdhury, R. D. Smith, A. D. Davidson, and B. B. Shank. 2001. Self-assembly and binding of a sorting nexin to sorting endosomes. J. Cell Sci. 114:1743-1756. [DOI] [PubMed] [Google Scholar]
- 25.Lee, J. J., G. Radice, C. P. Perkins, and F. Costantini. 1992. Identification and characterization of a novel, evolutionarily conserved gene disrupted by the murine H beta 58 embryonic lethal transgene insertion. Development 115:277-288. [DOI] [PubMed] [Google Scholar]
- 26.Marks, M. S. 1998. Determination of molecular size by sedimentation velocity analysis on sucrose gradients, p. 5.3.1-5.3.33. In J. S. Bonifacino, M. Dasso, J. B. Harford, J. Lippincott-Schwartz, and K. Yamada (ed.), Current protocols in cell biology. John Wiley & Sons, New York, NY.
- 27.Nakada-Tsukui, K., Y. Saito-Nakano, V. Ali, and T. Nozaki. 2005. A retromerlike complex is a novel Rab7 effector that is involved in the transport of the virulence factor cysteine protease in the enteric protozoan parasite Entamoeba histolytica. Mol. Biol. Cell 16:5294-5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nothwehr, S. F., S. A. Ha, and P. Bruinsma. 2000. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J. Cell Biol. 151:297-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nothwehr, S. F., and A. E. Hindes. 1997. The yeast VPS5/GRD2 gene encodes a sorting nexin-1-like protein required for localizing membrane proteins to the late Golgi. J. Cell Sci. 110:1063-1072. [DOI] [PubMed] [Google Scholar]
- 30.Oliviusson, P., O. Heinzerling, S. Hillmer, G. Hinz, Y. C. Tse, L. Jiang, and D. G. Robinson. 2006. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18:1239-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips, S. A., V. A. Barr, D. H. Haft, S. I. Taylor, and C. R. Haft. 2001. Identification and characterization of SNX15, a novel sorting nexin involved in protein trafficking. J. Biol. Chem. 276:5074-5084. [DOI] [PubMed] [Google Scholar]
- 32.Pons, V., F. Hullin-Matsuda, M. Nauze, R. Barbaras, C. Peres, X. Collet, B. Perret, H. Chap, and A. Gassama-Diagne. 2003. Enterophilin-1, a new partner of sorting nexin 1, decreases cell surface epidermal growth factor receptor. J. Biol. Chem. 278:21155-21161. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz, D. G., C. T. Griffin, E. A. Schneider, D. Yee, and T. Magnuson. 2002. Genetic analysis of sorting nexins 1 and 2 reveals a redundant and essential function in mice. Mol. Biol. Cell 13:3588-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seaman, M. N. 2004. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J. Cell Biol. 165:111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seaman, M. N. 2005. Recycle your receptors with retromer. Trends Cell Biol. 15:68-75. [DOI] [PubMed] [Google Scholar]
- 36.Seaman, M. N., E. G. Marcusson, J. L. Cereghino, and S. D. Emr. 1997. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 137:79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seaman, M. N., J. M. McCaffery, and S. D. Emr. 1998. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142:665-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi, H., R. Rojas, J. S. Bonifacino, and J. H. Hurley. 2006. The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat. Struct. Mol. Biol. 13:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddhanta, U., J. McIlroy, A. Shah, Y. Zhang, and J. M. Backer. 1998. Distinct roles for the p110alpha and hVPS34 phosphatidylinositol 3′-kinases in vesicular trafficking, regulation of the actin cytoskeleton, and mitogenesis. J. Cell Biol. 143:1647-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teasdale, R. D., D. Loci, F. Houghton, L. Karlsson, and P. A. Gleeson. 2001. A large family of endosome-localized proteins related to sorting nexin 1. Biochem. J. 358:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verges, M., F. Luton, C. Gruber, F. Tiemann, L. G. Reinders, L. Huang, A. L. Burlingame, C. R. Haft, and K. E. Mostov. 2004. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat. Cell Biol. 6:763-769. [DOI] [PubMed] [Google Scholar]
- 42.Wang, D., M. Guo, Z. Liang, J. Fan, Z. Zhu, J. Zang, X. Li, M. Teng, L. Niu, Y. Dong, and P. Liu. 2005. Crystal structure of human vacuolar protein sorting protein 29 reveals a phosphodiesterase/nuclease-like fold and two protein-protein interaction sites. J. Biol. Chem. 280:22962-22967. [DOI] [PubMed] [Google Scholar]
- 43.Zhong, Q., C. S. Lazar, H. Tronchere, T. Sato, T. Meerloo, M. Yeo, Z. Songyang, S. D. Emr, and G. N. Gill. 2002. Endosomal localization and function of sorting nexin 1. Proc. Natl. Acad. Sci. USA 99:6767-6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong, Q., M. J. Watson, C. S. Lazar, A. M. Hounslow, J. P. Waltho, and G. N. Gill. 2005. Determinants of the endosomal localization of sorting nexin 1. Mol. Biol. Cell 16:2049-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.