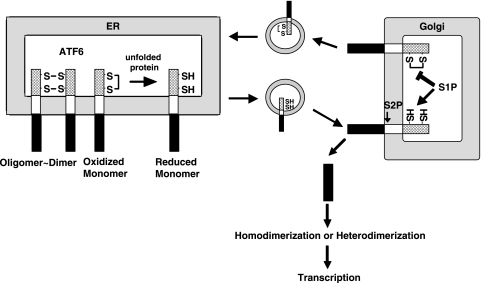
FIG. 10.
Model for regulation of ATF6 activation. ATF6 occurs in unstressed ER as the oxidized monomer, dimer, and oligomer due to intra- and intermolecular disulfide bridges formed in the luminal domain. Upon ER stress, ATF6 is reduced, and the reduced monomer ATF6 is transported to the Golgi apparatus to be cleaved by S1P and S2P. The N-terminal transcription factor domain liberated is homo- or heterodimerized and translocated into the nucleus to activate transcription of ER chaperone genes. ATF6 may move to the Golgi apparatus in the absence of ER stress but will return to the ER because oxidized ATF6 is resistant to cleavage by S1P. Thus, the cell processes only ATF6 which has experienced stress in the ER.