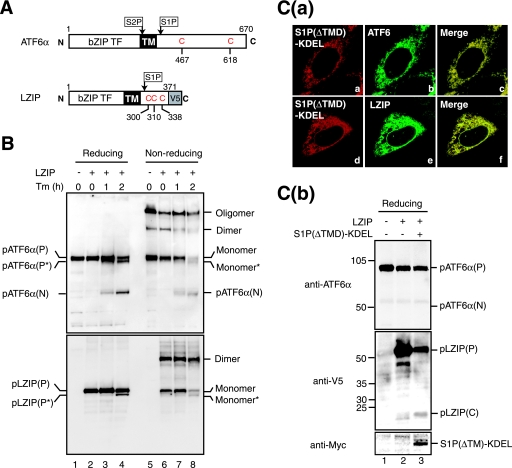
FIG. 9.
Effects of tunicamycin treatment and enforced expression of S1P in the ER on oligomeric status and cleavage of LZIP. (A) Schematic structures of ATF6α and LZIP tagged with the V5 epitope at the C terminus are shown. Both contain a single transmembrane domain (TM) in the middle of the respective protein, and their N-terminal bZIP transcription factor (TF) domains face the cytoplasm. Cleavage of ATF6α by S1P and S2P as well as that of LZIP by S1P was previously demonstrated. The positions of cysteine residues in their luminal regions are also indicated. (B) CHO cells transfected with (+) or without (−) a plasmid to express V5-tagged LZIP were treated with 2 μg/ml Tm for the indicated periods, and then cell lysates were prepared and analyzed as described for Fig. 3A(a) (without NEM) using anti-ATF6α (upper panel) or anti-V5 (lower panel) antibodies. Proteins marked by asterisks lack a carbohydrate moiety because of the action of Tm. (C) (a) CHO cells were cotransfected with plasmids to express myc-tagged S1P(ΔTMD)-KDEL and V5-tagged LZIP, and then immunostained with anti-myc (panels a and d), anti-ATF6α (panel b), or anti-V5 (panel e) antibodies. (b) CHO cells were mock transfected (lane 1), transfected with a plasmid to express V5-tagged LZIP alone (lane 2), or cotransfected with plasmids to express V5-tagged LZIP and myc-tagged S1P(ΔTMD)-KDEL (lane 3), and then cell lysates were prepared and analyzed by immunoblotting under reducing conditions using anti-ATF6α (upper panel), anti-V5 (middle panel), or anti-myc (lower panel) antibodies.