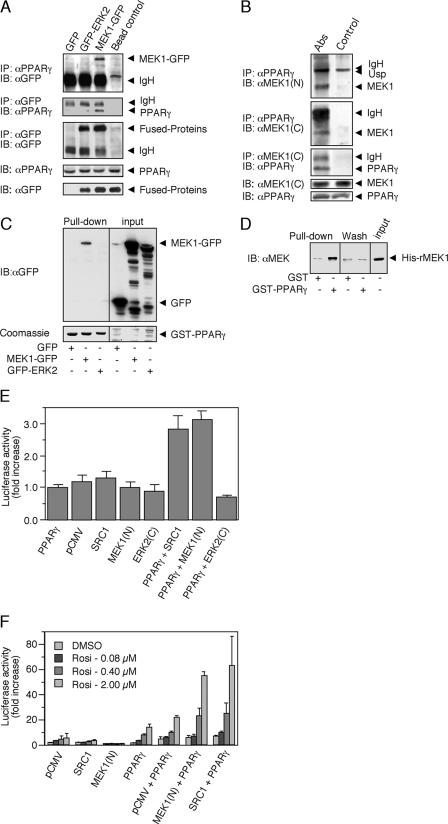
FIG. 3.
PPARγ association with MEK1. (A) CoIP. HEK-293 cells were transiently cotransfected with PPARγ1 and GFP control vector, GFP-ERK2, or MEK1-GFP expression plasmids. Cytosolic extracts were incubated with the indicated Abs conjugated to protein A/G-agarose beads or with beads alone, and then CoIP was performed. The amount of MEK1-GFP and GFP-ERK2 was determined by Western blotting (IB) with GFP Ab (first panel). Reverse CoIP with GFP Ab was analyzed by Western blotting with PPARγ Ab (second panel). The equal amount of GFP-ERK2 or MEK1-GFP was determined by IP and Western blotting with GFP Ab (third panel). The amounts of PPARγ and GFP proteins in the extracts were determined by Western blotting with PPARγ and GFP Ab (fourth and fifth panels). (B) CoIP. Cytosolic extracts from HEK-293 cells were incubated with PPARγ Ab or Ab against the C terminus of MEK1 [MEK1 (C)] conjugated to protein A/G-agarose beads or beads alone. IP was performed as described, and the amount of precipitated proteins was determined using Ab to the N terminus [MEK1 (N)] or C terminus of MEK1 and to PPARγ as indicated. The amounts of MEK1 and PPARγ in the extracts were determined by Western blotting with the appropriate Abs (fourth and fifth panels). Usp, unspecified band. (C) Cellular GST pull down. HEK-293 cells were transfected as for panel A, and their cytosolic extracts were incubated with GST-PPARγ. (D) In vitro GST pull down. Recombinant HIS-MEK1(FL) was incubated with GST-PPARγ or GST alone in the presence of 0.1% bovine serum albumin to avoid nonspecific interactions. (E and F) Interaction of MEK1 and PPARγ detected by mammalian two-hybrid analyses. Cells were cotransfected with a GAL4-UAS-reporter plasmid and GAL4-PPARγ-LBD together with pCMV-AD or fusions, in which the N terminus of MEK1(N), the central LXXLL domain of SRC1, or the inverted C-terminal domain of ERK2(C) were inserted downstream of the NF-κB activation domain. Graphs represent n-fold increases ± standard deviations of luciferase activity in the presence of elevating concentrations of rosiglitazone (n = 3). (E) Basal interactions in HEK-293 cells. (F) Ligand-driven interactions in HEK-293.