Abstract
Activation of extracellular signal-regulated protein kinase (ERK) has been implicated in proliferation as well as differentiation in a wide variety of cell types. Using B-cell-specific gene-targeted mice, we report here that in T-cell-dependent immune responses, ERK2 is required to generate efficient immunoglobulin G (IgG) production. In its absence, the proportion of antigen-specific surface IgG1-bearing cells and the subsequent number of IgG1 antibody-secreting cells were decreased, despite apparently unimpaired class switch recombination. Notably, this defect was countered by overexpression of the antiapoptotic factor Bcl-2. Together, our results suggest that ERK2 plays a key role in efficient generation of antigen-specific IgG-bearing B cells by promoting their survival.
Once B lymphocytes encounter antigens, a complex series of activation and maturation events drive the generation of antibody-secreting plasma cells and memory cells (31). In the case of immunization with T-cell-dependent (TD) antigens, cognate interaction between antigen-specific B cells and primed helper T cells triggers the B cells to proliferate in the periarterial lymphoid sheath in the spleen (17, 22). Some of the founder B cells then migrate to extrafollicular sites, where they ultimately grow plasmablasts to short-lived plasma cells, and the others form the germinal center (GC), which is necessary for efficient immunoglobulin (Ig) somatic hypermutation and affinity maturation, followed by the appearance of long-lived plasma cells (14, 18, 24, 25, 27, 41). The cell surface receptors responsible for such differentiation of B cells to plasma cells are well known. For instance, these include B-cell receptor (BCR), tumor necrosis factor family receptors (CD40 and BAFF receptor), and receptors for cytokines interleukin-4 (IL-4), IL-6, and IL-10 (10, 30, 36). By contrast, relatively little is known about their signaling cascades, which in turn culminate in B-cell expansion and differentiation into plasma cells.
A number of studies have implicated the Ras/MEK/extracellular signal-regulated protein kinase (ERK) pathway in early B-cell development as well as in memory antibody responses (7, 29, 32, 37, 40). Transgenic mice harboring a dominant inhibitory mutant of Ras manifested reduced numbers of pre-B cells and immature B cells, implying the importance of the Ras pathway in pre-BCR- and BCR-mediated cell fate decision (29). Moreover, by using the same transgenic mice, the Ras pathway has been recently demonstrated to participate in recruitment of high-affinity B cells into the memory compartment and in their terminal differentiation (40). These studies clearly suggest the importance of the Ras pathway in B-cell physiology. However, a role of ERK cannot be simply extrapolated from these data, because Ras is thought to exert its biological function through at least three effector molecules: ERK, phosphoinositide 3-kinase, and guanine nucleotide exchange factors for the Ras-related GTPase Ral (8, 19).
ERK1 and ERK2 constitute a focal point of mitogen-activated protein kinase (MAPK) pathway signaling in mammalian cells. These two highly homologous serine-threonine kinases are activated by tyrosine and threonine dual phosphorylation, thereby transmitting this activation to both cytoplasmic signaling complexes and nuclear transcription factors. ERK is likely to be the origin of a ramifying signal transduction program that affects many aspects of cellular responses (20). Indeed, in the case of B cells, the importance of ERK has been suggested by previous experiments using pharmacological inhibitors; MEK/ERK inhibitors partially impaired B-cell proliferation in response to BCR stimulation in vitro (32). However, its physiological function in B-cell development and activation still remains unclear. Thus, to approach this issue, we generated mice in which the ERK2 gene was deleted in a B-cell-specific manner. Analyses of these mice reveal that ERK2 contributes to efficient generation of antigen-specific IgG1-bearing B cells during TD antibody reactions, at least partly by providing a survival signal.
MATERIALS AND METHODS
Mice.
Generation of Mapk1flox/flox or Mapk1flox/+ mice, in which exon 3 of Mapk1 is flanked by LoxP sites, was described previously (11). These mice were crossed to Cd19Cre/+ mice (33) to generate Cd19Cre/+ Mapk1flox/flox mice. Age- and sex-matched 10- to 12-week-old Cd19Cre/+ Mapk1flox/flox and Cd19Cre/+ Mapk1+/+ mice were used for all experimental analysis, according to the guidelines established by the RIKEN animal committee.
Southern blot and Western blot analyses.
The purified genomic DNA was digested with SacI and subjected to Southern blot analysis to check the efficiency of deletion of Mapk1. For Western blot analysis, cells were lysed with lysis buffer (1% NP-40, 20 mM Tris-Cl [pH 8.0], 150 mM NaCl, 5 mM EDTA, and protease inhibitor cocktail) (Roche), and whole-cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. Anti-ERK (K-23) and anti-phospholipase C γ2 (Q-20) antibodies were purchased from Santa Cruz.
Immunization.
Mice were immunized with either 50 μg of TNP-Ficoll or 50 μg of alum-precipitated TNP-keyhole limpet hemocyanin (KLH) intraperitoneally (i.p.). For a recall response, 25 μg of TNP-KLH in phosphate-buffered saline (PBS) was administered i.p. without adjuvant 6 weeks after primary immunization. Sheep red blood cells (SRBC) were obtained from Nippon Bio-Test Laboratories Inc. Cells were washed three times with PBS before immunization, and 3 × 108 cells were injected i.p. per mouse.
Antibodies, cell preparations, and flow cytometry.
The following monoclonal antibodies (MAbs) were used in this study: anti-B220 (RA3-6B2), anti-CD43 (S7), anti-IgM (R6-60.2), anti-CD23 (B3B4), anti-CD5, anti-IgG1 (A85-1), anti-CD11b (M1/70), anti-CD11c (HL3), anti-Gr1 (RB6-8C5), anti-CD4 (GK1.5), anti-CD8a (53-6.7), anti-NK1.1 (PK136), anti-TER119 (Ly-76), and Fc block (2.4G2) (all from BD Bioscience); anti-IgD (11-26) (from Southern Biotechnology); and anti-AA4.1 and anti-F4/80 (BM8) (from e-Bioscience). Biotin-conjugated peanat agglutinin (PNA) was from Vector Laboratories. To generate biotin-conjugated TNP, TNP25-bovine serum albumin (BSA) was biotinylated using the Antibody Biotinylation kit (American Qualex). Splenic B cells, T cells, and dendritic cells were isolated with a MACS separation column (Miltenyi Biotech). For all except a few in vitro experiments, B cells from spleens were purified by depletion of non-B cells with anti-CD43 magnetic beads (Miltenyi Biotech). T cells and dendritic cells from spleens were purified positively with anti-CD90.2 and CD11c magnetic beads (Miltenyi Biotech), respectively. The purities of B-cell, T-cell, and dendritic cell fractions were >90%, >85%, and >80%, respectively, in each experiment. For detection of antigen-specific B cells, single-cell suspensions from spleens were incubated with cell-staining buffer (1× PBS, 3% BSA, 5 mM EDTA, and 0.01% NaN3) containing biotin-conjugated MAbs against CD4, CD8, F4/80, CD11b, CD11c, Gr-1, NK1.1, and TER119 and subsequently with iMAG streptavidin-conjugated microbeads (BD Bioscience). For enzyme-linked immunospot (ELISPOT) assays, single-cell suspensions from spleens were incubated with cell-staining buffer containing biotin-conjugated MAbs against CD4, CD8, F4/80, CD11c, NK1.1, and TER119 and subsequently with iMAG streptavidin-conjugated microbeads. For flow cytometry, cells were incubated with cell-staining buffer containing MAbs conjugated to fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll protein-Cy5.5, allophycocyanin, and biotin. Stained cells were analyzed using Cell Quest software on a FACSCalibur (BD Biosciences).
ELISA.
To measure total IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA Abs in sera of naïve mice and antigen-specific Abs in sera of immunized mice, enzyme-linked immunosorbent assays (ELISA) were performed. Briefly, sera were captured with plate-coated purified goat anti-mouse IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA (Southern Biotechnology) and detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA, respectively. For antigen-specific Abs, plates were coated with 10 μg/ml of TNP25 or TNP3-BSA and Abs were detected with HRP-conjugated goat anti-mouse IgM, IgG1, IgG2b, and IgG3. In all situations, wells were developed with the ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] liquid substrate system (Sigma) and the optical density was measured at 405 nm. Titers of antigen-specific Abs were determined by interpolation of the dilution factor to an optical density value in the linear range on a standard curve made by serial dilution of serum.
ELISPOT assays.
To detect antigen-specific antibody-secreting cells (ASCs), enzyme-linked immunospot (ELISPOT) assays were performed. Briefly, plates for ELISPOT assays (MultiScreen-HA; Millipore) were coated with 10 μg of TNP25-BSA. Cells depleted of non-B fractions in spleen were plated at 106 cells per well and incubated at 37°C for 5 h. ASCs were detected with HRP-conjugated goat anti-mouse IgM or IgG1 (Southern Biotechnology), and wells were developed with AEC substrate reagent set for ELISPOT (BD Bioscience).
Reverse transcription-PCR (RT-PCR).
Splenic B cells were treated with anti-CD40 MAb (clone HM40-3; BD Bioscience) plus recombinant mouse IL-4 (R&D) for 4 days. Total RNA was purified with the TRIzol reagent (Invitrogen) and subjected to cDNA synthesis using SuperScript first-strand synthesis system (Invitrogen) according to the manufacture's instructions. PCR was performed on 25 ng and subsequent threefold dilutions of the cDNA by using specific primers for γ1 germ line transcripts (GLTs), γ1 postswitch transcripts (PSTs), and activation-induced cytidine deaminase (AID) as described previously (28). Amplification of G3PDH (glyceraldehyde-3-phosphate dehydrogenase) was performed as an internal control. PCR conditions were 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min for 35 cycles (γ1 GLT, γ1 PST, and AID) or 30 cycles (G3PDH). Primers used were as follows: γ1 GLT, 5′-GGCCCTTCCAGATCTTTGAG-3′ and 5′-GGATCCAGAGTTCCAGGTCAC-3′; γ1 PST, 5′-CTCTGGCCCTGCTTATTGTTG-3′ and 5′-GGATCCAGAGTTCCAGGTCAC-3′; AID, 5′-GGCTGAGGTTAGGGTTCCATCTCAG-3′ and 5′-GAGGGAGTCAAGAAAGTCACGCTGGA-3′; and G3PDH, 5′-CTGGCCAAGGTCATCCATGAC-3′ and 5′-AGGTCCACCACCCTGTTGCTG-3′.
DC-PCR analysis of Sμ-Sγ1 genomic DNA rearrangements.
Digestion-circularization PCR (DC-PCR) was performed as described previously (3). Briefly, splenic B cells were treated with anti-CD40 MAb plus recombinant mouse IL-4 for 4 days, and genomic DNA was extracted. Two micrograms of genomic DNA was digested overnight with EcoRI, treated with RNase A, and purified. EcoRI-digested DNA was ligated at 16°C using a DNA ligation kit (Takara) according to the manufacturer's instruction. Following ligation, purified ligated DNA was subjected to PCR. PCR conditions were as follows: 1 cycle at 94°C for 6 min; 5 cycles at 94°C for 20 s, 58°C for 1 min, and 72°C for 2 min; 30 cycles at 94°C for 20 s, 65°C for 1 min, and 72°C for 2 min; and a final cycle at 72°C for 7 min. Primers sequences for Sμ-Sγ1 were 5′μ (5′-GGCCGGTCGACGGAGACCAATAATCAGAGGGAAG-3′) and 3′γ1 (5′-GCGCCATCGATGGAGAGCAGGGTCTCCTGGGTAGG-3′), and those for nAChR were A1 (5′-GGCCGGTCGACAGGCGCGCACTGACACCACTAAG-3′) and A2 (5′-GCGCCATCGATGGACTGCTGTGGGTTTCACCCAG-3′).
CFSE labeling and cell division tracking.
Splenic naïve B cells were labeled with RPMI 1640 containing 5 μM 5 (and 6-)-carboxyfluorescein diacetate succinimidyl (CFSE) at 37°C for 10 min at a cell concentration of 1 × 107 cells/ml. The CFSE-labeled cells were subsequently washed with RPMI complete medium (RPMI 1640 supplemented with 10% fetal calf serum and 50 μM β-mercaptoethanol) and then stimulated with anti-CD40 MAb plus recombinant mouse IL-4 for 4 days. Progression of cell division and surface IgG1 expression were analyzed by flow cytometry.
BrdU labeling.
Mice were immunized with 50 μg of alum-precipitated TNP-KLH. Seven days later, mice were administered bromodeoxyuridine (BrdU) (2 mg in PBS per mouse) by i.p. injection 5 h before sacrifice. Spleens were harvested, and single-cell suspensions were incubated with cell-staining buffer containing biotin-conjugated MAbs against CD4, CD8, F4/80, CD11b, CD11c, Gr-1, NK1.1, and TER119 to deplete non-B-cell fractions and subsequently with iMAG streptavidin-conjugated microbeads. Purified B cells were further incubated with biotin-conjugated TNP and subsequently with iMAG streptavidin-conjugated microbeads. Purified TNP-binding B cells were stained with fluorescein-conjugated Abs, and subsequently detection of BrdU-labeling cells was performed using a BrdU flow kit (BD Bioscience) according to the manufacturer's instructions.
Retroviral transduction of bone marrow cells and generation of bone marrow chimeras.
The cDNA encoding mouse Bcl-2 was cloned into an murine stem cell virus-based retroviral vector containing enhanced green fluorescent protein (GFP) cDNA as an expression marker downstream of the internal ribosomal entry site. Generation of viral supernatants, retroviral infection into bone marrow cells, and production of bone marrow chimeras were performed as described previously (13). Briefly, plasmids were transiently transfected into Plat E packaging cells, and 3 days later the supernatants were collected. Cycling bone marrow progenitors were enriched by injecting Cd19Cre/+ Mapk1flox/flox or wild type littermate mice with 5-fluorouracil (150 mg/kg body weight) (Sigma) in PBS 4 days prior to harvest of bone marrow from femurs and tibias. Bone marrow cells were cultured in bone marrow cell culture medium (DMEM supplemented with 15% fetal calf serum, murine stem cell factor, murine thrombopoietin, murine Flt3 ligand, murine IL-6, and murine IL-3) (the working concentration of all cytokines was 20 ng/ml, and all were purchased from R&D). After 2 days, cultured bone marrow cells were collected, suspended at a density of 1 × 106 cells per 500 μl of bone marrow cell culture medium, and mixed with 500 μl of virus supernatants containing Polybrene (final concentration, 6 μg/ml; Sigma), and then these were spin infected at 2,000 rpm for 90 min at 32°C in 24-well plates and cultured for 3 days. On the fourth day of culture, 2 × 106 infected donor bone marrow cells were injected intravenously into recipient wild-type mice pretreated with gamma irradiation at a dose of 8.5 Gy.
RESULTS
Generation of B-cell-specific ERK2-deficient mice.
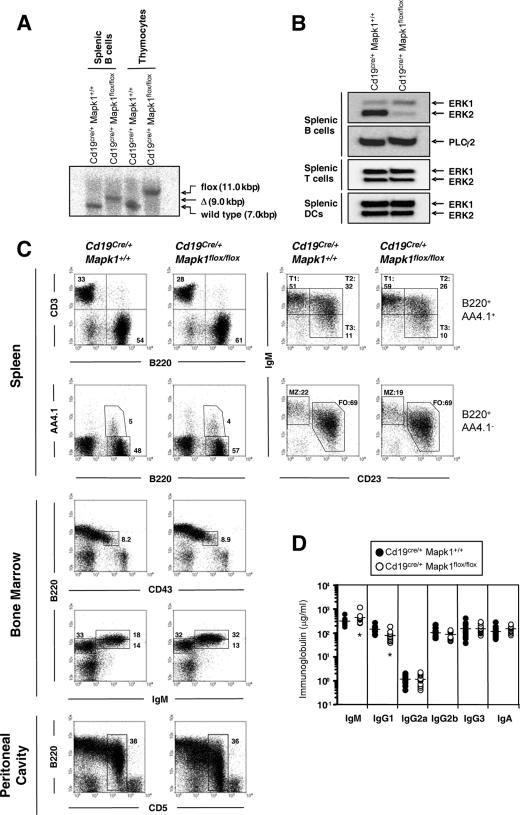
In our preliminary experiments using semiquantitative RT-PCR analysis, the expression level of ERK2 in splenic B cells was about twofold higher than that of ERK1 (data not shown). Thus, in this study, as a first step to elucidate ERK function, we focused on clarifying the in vivo function of ERK2. Targeted disruption of ERK2 results in embryonic lethality due to defective placental development (11, 35). Therefore, we established B-cell-specific ERK2-deficient mice by using the Cre-LoxP system, in which mice harboring a flox allele of the Mapk1 (= ERK2) gene (Mapk1flox/flox) were intercrossed with mice expressing Cre recombinase under the control of the Cd19 promoter (Cd19Cre/+) (33). Efficient Cre-mediated deletion of the ERK2 gene was demonstrated in splenic B cells from Cd19Cre/+ Mapk1flox/flox mice by Southern blotting (Fig. 1A and data not shown). Efficient elimination of ERK2 protein in splenic B cells of Cd19Cre/+ Mapk1flox/flox mice was also confirmed by immunoblotting. Compared with the control B cells, the mutant B cells exhibited slightly higher expression of ERK1 protein, probably due to coping with the ERK2 deficits (Fig. 1B). As expected, the same amount of ERK2 protein was observed in splenic T cells and dendritic cells of Cd19Cre/+ Mapk1flox/flox mice as in those of Cd19Cre/+ Mapk1+/+ mice (Fig. 1B). Collectively, specific deletion of MAPK1 was achieved efficiently in Cd19Cre/+ Mapk1flox/flox mice.
FIG. 1.
B-cell development in B-cell-specific ERK2-deficient mice. (A) Southern blot analysis of SacI-digested genomic DNAs from splenic B cells and thymocytes. Note the specific deletion of the flox allele in splenic B cells but not in thymocytes of Cd19Cre/+ Mapk1flox/flox mice. (B) Western blot analysis of whole-cell lysates from splenic B cells, T cells, and dendritic cells by using an antibody specific for ERK1 and ERK2. The blot of phospholipase C γ2 is shown as an internal control in B cells. (C) B-cell development in bone marrow, spleen, and peritoneal cavity of Cd19Cre/+ Mapk1+/+ and Cd19Cre/+ Mapk1flox/flox mice. Results are representative of six independent experiments. (D) Serum immunoglobulin titers. Resting levels of each immunoglobulin isotype in the sera were estimated by ELISA. There were 13 mice in each group. Significant differences between Cd19Cre/+ Mapk1flox/flox and Cd19Cre/+ Mapk1+/+ mice as calculated by Student's t test are indicated (*, P < 0.05).
Impaired TD immune responses in Cd19Cre/+ Mapk1flox/flox mice.
B-cell development in Cd19Cre/+ Mapk1flox/flox mice was first analyzed by flow cytometry. As shown in Fig. 1C and Table 1, we did not observe significant differences in the numbers and proportions of pro-, pre-, immature, transitional, follicular, and marginal zone B cells in the bone marrow and spleens of Cd19Cre/+ Mapk1+/+ and Cd19Cre/+ Mapk1flox/flox mice, though slightly increased numbers and percentages of bone marrow recirculating B cells were observed. Moreover, in these two types of mice, percentages of peritoneal B1-a B cells did not differ significantly. Levels of IgG2a, IgG2b, IgG3, and IgA in sera of Cd19Cre/+ Mapk1flox/flox mice were almost the same as in Cd19Cre/+ Mapk1+/+ mice; however, the concentrations of serum IgM and IgG1 were significantly higher and lower, respectively, in Cd19Cre/+ Mapk1flox/flox mice (Fig. 1D).
TABLE 1.
B-cell numbers in bone marrow and spleen in Cd19Cre/+ Mapk1+/+ and Cd19Cre/+ Mapk1flox/flox mice
| Cells | No. (106)b in:
|
|
|---|---|---|
| Cd19Cre/+Mapk1+/+ mice | Cd19Cre/+Mapk1flox/flox mice | |
| Bone marrowa | ||
| Pro B (B220lo IgM− CD43+) | 0.206 ± 0.042 | 0.359 ± 0.119 |
| Pre B (B220lo IgM− CD43−) | 1.987 ± 0.859 | 2.112 ± 0.529 |
| Immature B (B220lo IgM+) | 0.935 ± 0.217 | 1.258 ± 0.506 |
| Recirculating B (B220hi IgM+) | 0.722 ± 0.213 | 1.670 ± 0.860 |
| Spleen | ||
| T1 (B220+AA4.1+ IgMhi CD23−) | 1.339 ± 0.377 | 1.546 ± 0.764 |
| T2 (B220+AA4.1+ IgMhi CD23+) | 0.504 ± 0.146 | 0.690 ± 0.305 |
| T3 (B220+AA4.1+ IgMlo CD23+) | 0.250 ± 0.098 | 0.314 ± 0.133 |
| Follicular (B220+ AA4.1− IgMloCD23+) | 26.02 ± 7.611 | 31.54 ± 7.607 |
| Marginal zone (B220+ AA4.1− IgMhi CD23−) | 7.960 ± 3.162 | 7.309 ± 2.278 |
Bone marrow cells were obtained from two femurs and tibias.
Cell number was calculated on the basis of total cell count and flow cytometric analysis. Data are shown as averages and standard deviations from six mice.
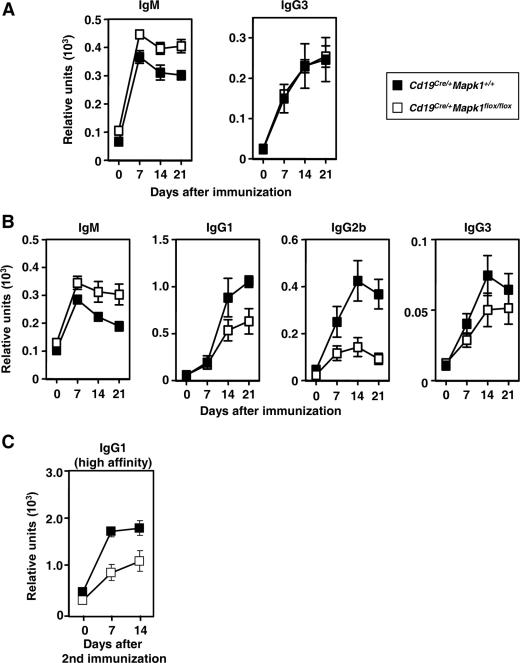
To assess the ability of Cd19Cre/+ Mapk1flox/flox mice to mount immune responses against antigens, we investigated T-cell-independent type 2 (TI-II) and TD immune responses. When immunized with TI-II antigens (TNP-Ficoll), the TNP-specific IgM, but not IgG3, response in Cd19Cre/+ Mapk1flox/flox mice was significantly increased compared with that in control mice (Fig. 2A).
FIG. 2.
TD immune responses are defective in Cd19Cre/+ Mapk1flox/flox mice. Mice were immunized with TNP-Ficoll (A) or TNP-KLH in alum (B and C). Sera were analyzed by ELISA for TNP-specific IgM and IgG3 (A) or TNP-specific IgM, IgG1, IgG2b, and IgG3 (B) by using TNP25-BSA as a capturing antigen. A secondary immunization with TNP-KLH without adjuvant in PBS was given 42 days after primary immunization (C). Collected sera from four mice were measured for high-affinity anti-TNP IgG1 by using TNP3-BSA as a capturing antigen. For all panels, each time point represents the average and standard error of the mean for four mice.
When immunized with TD antigens (TNP-KLH), titers of TNP-specific IgM were somewhat elevated in Cd19Cre/+ Mapk1flox/flox mice compared with Cd19Cre/+ Mapk1+/+ mice, as for TI-II immune responses (Fig. 2B). In contrast, Cd19Cre/+ Mapk1flox/flox mice showed a reduction in TNP-specific IgG of all subclasses tested (Fig. 2B). TNP3-BSA as a capturing antigen can detect IgG1 Abs with higher affinity. The ratio of anti-TNP IgG1 with higher affinity to total anti-TNP IgG1 was not different in Cd19Cre/+ Mapk1flox/flox and Cd19Cre/+ Mapk1+/+ mice during the primary response (data not shown), suggesting that affinity maturation occurs normally in the absence of ERK2. A secondary IgG1 response, as assessed by titers of anti-TNP IgG1 with higher affinity, was defective in Cd19Cre/+ Mapk1flox/flox mice (Fig. 2C). Taken together, these results demonstrate that ERK2 is required to mount efficient primary and secondary IgG responses to TD antigens in vivo.
Reduced proportion of antigen-specific IgG1-bearing B cells in Cd19Cre/+ Mapk1flox/flox mice in TD responses.
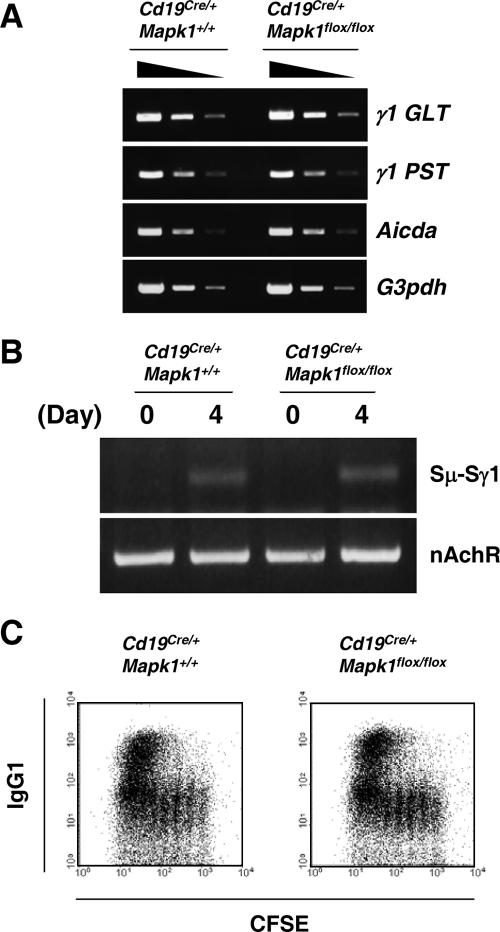
Having demonstrated the importance of ERK2 in primary IgG1 antibody production in TD responses, we wished to address the underlying mechanism. The impaired IgG1 response in Cd19Cre/+ Mapk1flox/flox mice could be accounted for by the following possibilities: (i) inefficient class switching to IgG1, (ii) inefficient proliferation and/or persistence of surface IgG1-bearing B cells, or (iii) inefficient differentiation to plasma cells. To examine these possibilities, we first compared the class switch capability. Splenic B cells were prepared from Cd19Cre/+ Mapk1flox/flox and Cd19Cre/+ Mapk1+/+ mice and stimulated in vitro with anti-CD40 Ab and IL-4. Flow cytometric analysis demonstrated that similar percentages of Cd19Cre/+ Mapk1flox/flox B cells and control B cells possessed IgG1 on their cell surfaces (Fig. 3C). Moreover, similar levels of γ1 GLTs and γ1 PSTs as well as digestion circularization-PCR products were detected (Fig. 3A and B). Based on these observations, we conclude that class switching in general is unaffected in Cd19Cre/+ Mapk1flox/flox mice.
FIG. 3.
In vitro class switch recombination occurs normally in ERK2-deficient B cells. Splenic B cells were purified and stimulated with 2 μg/ml of anti-CD40 MAb plus 10 ng/ml of mouse IL-4 for 4 days. (A) Total RNA was extracted, and γ1 germ line transcript (γ1GLT), γ1 postswitch transcript (γ1PST), AID (Aicda), and G3PDH (G3pdh) were detected by semiquantitative RT-PCR from threefold dilutions of each cDNA. (B) Genomic DNA was extracted and analyzed by DC-PCR. (C) CFSE-labeled B cells were stimulated and analyzed by flow cytometry to detect the progression of cell division and IgG1-bearing B cells concurrently. All results are representative of at least two independent experiments.
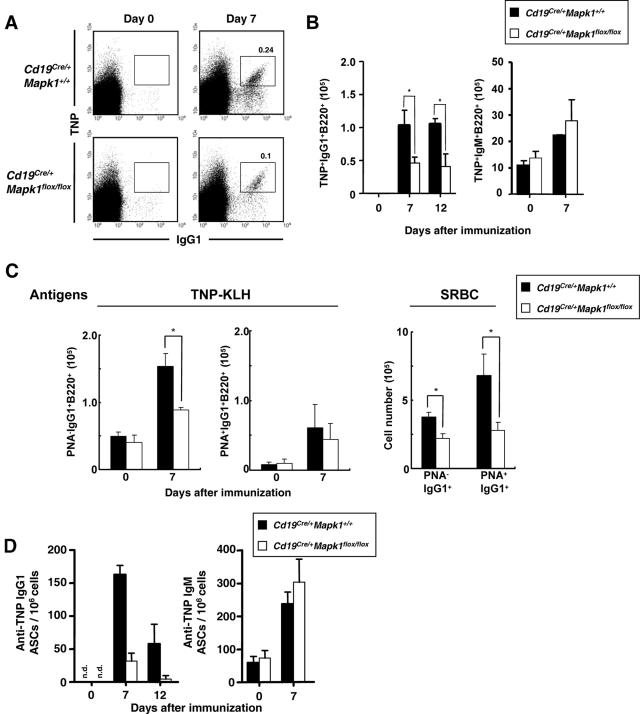
Next, to determine whether the impaired IgG1 antibody response is due to defective B-cell proliferation and/or persistence, antigen-specific IgG1 B cells were tracked by flow cytometry after immunization with TD antigens. At day 7, compared with that in Cd19Cre/+ Mapk1+/+ mice, the number of TNP-specific IgG1-bearing B cells was significantly decreased in Cd19Cre/+ Mapk1flox/flox mice (Fig. 4A and B). In contrast to IgG1-bearing B cells, the number of TNP-specific IgM B cells was equivalent in Cd19Cre/+ Mapk1+/+ and Cd19Cre/+ Mapk1flox/flox mice (Fig. 4B). The TNP-specific IgG1-bearing B cells are likely a mixture of cells derived from extrafollicular and GC responses. Hence, to determine which compartment is affected in the absence of ERK2, after mice were immunized with TNP-KLH (Fig. 4C, left panel) or SRBC (Fig. 4C, right panel), IgG1-bearing B cells were stained by PNA, a GC marker. As shown in Fig. 4C, the number of IgG1+ PNA− cells (extrafollicular B cells) was decreased in Cd19Cre/+ Mapk1flox/flox mice after both immunizations, and the number of IgG+ PNA+ cells (GC B cells) also was decreased in Cd19Cre/+ Mapk1flox/flox mice after the SRBC immunization. Thus, these data indicate that ERK2 is required for efficient generation of IgG1-bearing B cells during the primary TD immune response, probably through both extrafollicular and GC processes.
FIG. 4.
Cd19Cre/+ Mapk1flox/flox mice exhibit lower numbers of antigen-specific IgG1 B cells during early TD immune responses. (A) Mice were immunized with TNP-KLH in alum, and 7 days later splenic B cells were purified and analyzed by flow cytometry. Plots depicting TNP versus IgG1, gated on B220+ cells, are shown. Percentages of TNP+ IgG1+ cells in purified B cells are shown. Data are representative of four to six independent experiments. (B) Kinetics of antigen-specific B cells in spleen after primary immunization. The calculated numbers of TNP-binding IgG1+ B220+ cells and TNP-binding IgM+ B220+ cells in a spleen are shown. TNP-binding IgG1+ B220+ cells cannot be detected in unimmunized mice. The plots show the average and standard error of the mean from three to six each mice for each time point. Significant differences between Cd19Cre/+ Mapk1flox/flox and Cd19Cre/+ Mapk1+/+ mice as calculated by Student's t test are indicated (*, P < 0.05). (C) B cells were purified from spleens of mice immunized with TNP-KLH or SRBC on day 7 or 6 after immunization, respectively. Cells stained with PNA and anti-IgG1 antibody were analyzed by flow cytometry, and the number of cells in a spleen was calculated. In the case of SRBC immunization, the only data for day 6 after immunization are shown. Means ± standard errors of the means of numbers of IgG1+ PNA− and IgG1+ PNA+ cells obtained from three mice are shown. Significant differences between Cd19Cre/+ Mapk1flox/flox and Cd19Cre/+ Mapk1+/+ mice as calculated by Student's t test are indicated (*, P < 0.05). (D) Mice were immunized with TNP-KLH in alum, and spleens were harvested on the indicated days. Non-B cells in spleen were depleted as described in Materials and Methods, and the remaining B cells were analyzed by ELISPOT assay to detect TNP-specific IgG1 or IgM ASCs. The plots show the average and standard error of the mean from three to six mice for each time point. n.d., not detected.
To further determine the numbers of IgG1 ASCs, we performed ELISPOT assays. Consistent with the decreased generation of antigen-specific IgG1-beraing B cells in Cd19Cre/+ Mapk1flox/flox mice, a decreased number of TNP-specific IgG1 ASCs was observed in spleens of the mutant mice over 7 to 12 days after immunization with TNP-KLH (Fig. 4D, left panel). On the other hand, the number of TNP-specific IgM ASCs was marginally increased in Cd19Cre/+ Mapk1flox/flox mice compared with Cd19Cre/+ Mapk1+/+ mice (Fig. 4D, right panel).
Impaired generation of antigen-specific IgG1-bearing cells in Cd19Cre/+ Mapk1flox/flox mice can be countered by overexpression of Bcl-2.
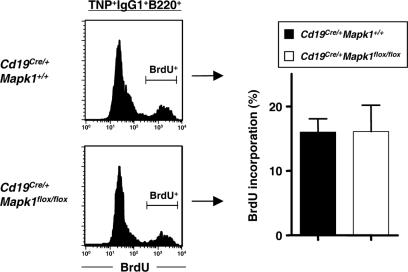
The defective generation of antigen-specific IgG1-bearing cells in Cd19Cre/+ Mapk1flox/flox mice might be the result of lower cell proliferation, of increased loss of IgG1-bearing B cells during TD immune responses, or of both. To verify the former possibility, we determined the proliferative activity of antigen-specific IgG1 B cells by analyzing cell cycling in the two sets of mice. Cd19Cre/+ Mapk1+/+ and Cd19Cre/+ Mapk1flox/flox mice were immunized with TNP-KLH, and then 7 days later, these mice were administered BrdU intraperitoneally for 5 h before sacrifice. The frequencies of BrdU-incorporated cells in TNP-specific IgG1 B cells in spleen were almost equivalent in Cd19Cre/+ Mapk1+/+ and Cd19Cre/+ Mapk1flox/flox mice (Fig. 5). This result suggests that ERK2 contributes little to proliferation of antigen-specific IgG1 B cells during early TD immune responses.
FIG. 5.
Normal proliferation capacity of antigen-specific IgG1 B cells during early TD immune responses in Cd19Cre/+ Mapk1flox/flox mice. Mice were immunized with TNP-KLH in alum and 7 days later administered BrdU (2 mg/mouse). Purified TNP-binding IgG1 B cells from spleen were analyzed by flow cytometry. Histograms (left) are representative of three independent experiments and, the graphs (right) show the average percentages and standard errors of the means of BrdU+ cells among TNP+ IgG1+ B220+ cells from three independent experiments.
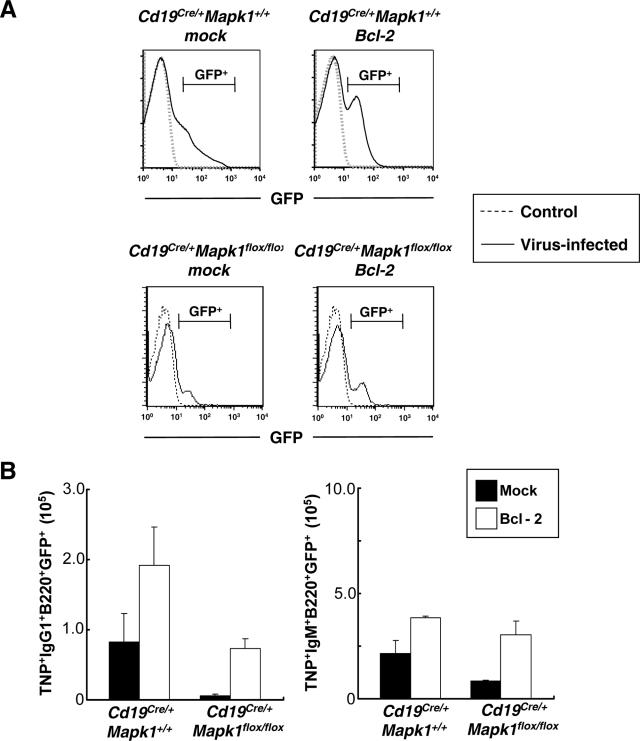
The above result, together with previous evidence that ERK participates in cell survival in other cell types (21, 23), prompted us to consider the possibility that ERK2 deficiency might cause cell death of IgG1-bearing cells during TD responses. This possibility can be tested by examining whether overexpression of Bcl-2, which is well known to inhibit cell death, might rescue the decrease of IgG1-bearing cells in ERK-2 deficient mice. We introduced Bcl-2 into bone marrow cells from Cd19Cre/+ Mapk1+/+ and Cd19Cre/+ Mapk1flox/flox mice by retroviral infection and then transferred them into lethally irradiated littermate wild-type control mice (Fig. 6). The reconstituted mice were immunized with TNP-KLH. Seven days later, splenic B cells were prepared, and then antigen-specific B cells among GFP-positive cells were analyzed by flow cytometry. There was about a 10-fold increase of TNP-specific IgG1 B cells in Cd19Cre/+ Mapk1flox/flox/Bcl-2-reconstituted mice compared with mock-reconstituted mice, while the increase in numbers of antigen-specific IgM cells was only threefold (Fig. 6B). In mice reconstituted with Cd19Cre/+ Mapk1+/+ bone marrow cells, both antigen-specific IgM and IgG1 cells are increased about twofold by overexpression of Bcl-2. Overexpression of Bcl-2 somehow enhances the proportion of antigen-specific IgM B cells under our experimental conditions (Fig. 6B, right panel), consistent with a previous report (38). Nevertheless, since a more robust increase of IgG1-bearing B cells was observed in Cd19Cre/+ Mapk1flox/flox/Bcl-2-reconstituted mice, we conclude that overexpression of Bcl-2 also participates in suppression of the defective generation of antigen-specific IgG1-bearing B cells deficient in ERK2.
FIG. 6.
Impaired generation of antigen-specific IgG1-bearing cells in Cd19Cre/+ Mapk1flox/flox mice can be countered by overexpression of Bcl-2. Bone marrow cells derived from Cd19Cre/+ Mapk1flox/flox and Cd19Cre/+ Mapk1+/+ mice were retrovirally infected and transferred intravenously into lethally irradiated wild-type mice as recipients. (A) Frequency of GFP-positive cells. Ten weeks after bone marrow transfer, peripheral blood of reconstituted mice was collected and GFP-positive B220+ cells were evaluated by flow cytometry. Histograms represent results from one of four independent experiments. GFP-positive cells: Cd19Cre/+ Mapk1+/+-mock, 20.1% ± 2.4%; Cd19Cre/+ Mapk1+/+-Bcl-2, 21.4% ± 4.9%; Cd19Cre/+ Mapk1flox/flox-mock, 16.7% ± 1.5%; Cd19Cre/+ Mapk1flox/flox-Bcl-2, 20.7% ± 5.7%. (B) Ten weeks after bone marrow transfer, mice were immunized with TNP-KLH in alum, and then 7 days later, the numbers of TNP+ IgG1+ GFP+ B220+ and TNP+ IgM+ GFP+ B220+ cells in a spleen were analyzed as described for Fig. 4. The plots show the averages and standard errors of the means from at least three independent experiments.
DISCUSSION
From a growing body of previous findings, the ERK pathway has been implicated in the control of various cellular processes, such as proliferation, differentiation, survival, and apoptosis (6). However, its physiological functions in B cells remain to be clarified. Here we focus on the in vivo role of ERK2 in B cells.
Overall, B-cell development takes place normally in Cd19Cre/+ Mapk1flox/flox mice. By contrast, previous in vitro experiments using pharmacological inhibitors demonstrated involvement of the ERK pathway in promoting pre-BCR- and BCR-mediated proliferation, which was thought to contribute to expansion of pre-BCR- and BCR-expressing populations in vivo (7, 32, 37). Hence, one straightforward explanation for the apparently normal B-cell development in Cd19Cre/+ Mapk1flox/flox mice is that remaining ERK activity, such as that derived from ERK1 and/or from incomplete deletion of ERK2 particularly at the pro-B stage (data not shown), could be sufficient for progression through the pre-B-cell stage as well as maintenance of the mature B-cell pool.
Although typical T-cell-derived signals are not required for TI-II responses, B cells responding to TI-II antigens receive costimulatory signals through innate immune receptors, such as complement receptors and Toll-like receptors (TLRs), that cooperate with signals through the BCR. In this regard, we observed that upon costimulation with TLR9 and BCR, in vitro differentiation to IgM-secreting plasma cells was increased in ERK2-deficient B cells (data not shown). Thus, it is likely that loss of ERK2 alters the signals through TLRs and the BCR, thereby leading to generation of larger amounts of TNP-specific IgM in response to TI-II antigens. This interpretation might be also supported by previous studies using the soluble hen egg lysozyme (HEL) and anti-HEL BCR model. Self-reactive anti-HEL BCRs on anergic B cells activate tyrosine kinase signaling poorly, but these self-reactive BCRs continue to activate the ERK pathway that contributes to blocking of TLR9-induced differentiation into plasma cells (12, 34).
In response to TD antigens, Cd19Cre/+ Mapk1flox/flox mice also showed somewhat increased amounts of TNP-specific IgM, which could be accounted for by the inhibitory role of ERK2 in differentiation into IgM-secreting cells, such as has been speculated in the case of TI-II responses. Alternatively, given the evidence that spleen has a limited capacity to sustain late plasmablast and plasma cell survival (39), the lower proportion of antigen-specific IgG ASCs in Cd19Cre/+ Mapk1flox/flox mice might reciprocally enhance the availability of this survival signal for antigen-specific IgM plasmablast and plasma cells in the spleen, thereby leading to hyper-IgM responses.
Despite an increase of IgM in TD responses, Cd19Cre/+ Mapk1flox/flox mice displayed an impaired IgG response to TD antigens. Initial T-B interactions are likely to occur normally in Cd19Cre/+ Mapk1flox/flox mice during the TD immune responses. This is supported by two lines of evidence. First, CD86 (B7-2) and class II molecules, both of which are required for cognate B-T interactions and subsequent lymphocyte activation, were normally up-regulated by BCR or CD40 stimulation in ERK2-deficient B cells (data not shown). Second, overall GC formation is not severely impaired in ERK2-deficient mice (data not shown), though IgG-expressing GC B cells were slightly decreased (Fig. 4C). The low IgG1 response is not also due to impaired isotype class switching, because Ig isotype class switching occurred normally in ERK2-deficient B cells after they were induced by anti-CD40 Ab together with IL-4 in vitro (Fig. 3). Thus, the impaired IgG1 response in Cd19Cre/+ Mapk1flox/flox mice is most likely caused by inefficient proliferation or survival of antigen-specific IgG1-bearing B cells and/or impaired differentiation into IgG1-secreting cells. Among these possibilities, our data highly suggest that ERK2 deficiency could cause a survival defect in antigen-specific IgG1-bearing cells, thereby leading to their inefficient generation during primary TD responses. In regard to the survival defect, we could not detect a significant difference in apoptosis in situ in mutant versus control mice by using the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling method (data not shown). We speculate that once IgG1-bearing cells undergo apoptosis in TD responses, these apoptotic cells can be promptly cleaned up by surrounding macrophages, thereby hindering our detection. Alternatively, this might simply be due to insufficient sensitivity of our detection system. The importance of a survival signal for an efficient IgG1 response was also highlighted by recent analysis using CD45-deficient B cells (15). CD45-deficient B cells failed to induce the efficient antigen-specific IgG1 response, and this defective response was countered by enforced expression of Bcl-xL.
Recent data obtained by using mice harboring IgM and IgG transgenes, both of which are specific for HEL, demonstrate the existence of functional differences between B cells expressing IgM and IgG BCRs in TD responses (26). B cells expressing IgM BCRs proliferated extensively after immunization with HEL, but their numbers in the spleen rapidly fell. In contrast, B cells expressing IgG BCRs proliferated extensively and persisted in the spleen. Our study might offer an explanation for the augmented persistence of IgG-bearing cells. IgG1-expressing B cells may be equipped with the ERK2-mediated survival signal, which in turn allows them to persist in the spleen, thereby inducing a more robust response. By contrast, in IgM-expressing B cells, ERK2 might not play such a role. Alternatively, in IgM-expressing B cells, the ERK2 activation might be weak, thereby not being sufficient to generate a survival signal in these cells. Indeed, a previous report showed that the IgG BCR, compared with the IgM BCR, can evoke stronger ERK activation and that this heightened activity of the IgG BCR is conferred by the cytoplasmic tail of the IgG heavy chain (42). Thus, it is reasonable to anticipate that the IgG BCR evokes robust ERK activation, which in turn contributes to cell survival and subsequent IgG responses.
While we have now shown that ERK2 deficiency inhibits a primary IgG1 response, the previous study using a dominant inhibitory mutant of Ras showed an apparently normal primary IgG1 response after immunization with TD antigens (40). Ras is well known to exert its biological function through guanine nucleotide exchange factors for Ral, in addition to ERK (43). Given the evidence that Ral is involved in receptor down-regulation (16, 44), the phenotype of mice harboring the mutant Ras might be manifested as a net outcome of its inhibitory impact on both ERK signaling and receptor down-regulation. Thus, the enhanced expression of stimulatory receptors on the B-cell surface might compensate for the loss of ERK, thereby generating a normal IgG1 response in the mutant mice.
Although this study has not directly addressed the issue of how the ERK2 cascade supports survival of IgG1-expressing B cells, several possibilities can be envisaged from previous studies. One possibility is that ERK2 might modulate expression and/or activity of the proapoptotic BH3-only proteins such as Bim in IgG1-bearing B cells. In the case of Bim, it binds to Bcl-2 by BCR cross-linking (5), thereby releasing the proapoptotic protein Bax or Bak (4). Countering this proapoptotic action, the ERK activity is thought to phosphorylate Bim, which targets it for ubiquitination and proteosomal degradation (21, 23). A second possibility is that ERK2 might phosphorylate caspase-9 and inhibit its activity, thereby promoting IgG1-bearing B-cell survival (1). Finally, a third possibility is that ERK2 might exert its survival function through transcriptional events, because it has been reported that the ERK pathway regulates expression of transcription factors such as Egr1 and Id3 (2, 9, 32). Future studies will be required to define the mechanisms of ERK2-induced survival in IgG1-bearing B cells.
Acknowledgments
We thank members of the Kurosaki laboratory for helpful discussion. We also thank T. Kitamura for providing Plat E cells and K. Kinoshita and H. Nagaoka for providing the protocol for DC-PCR.
This work was supported by grants to T.K. from the Ministry of Education, Science, Sport, and Culture of Japan and from the Uehara Memorial Foundation.
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Allan, L. A., N. Morrice, S. Brady, G. Magee, S. Pathak, and P. R. Clarke. 2003. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat. Cell. Biol. 5:647-654. [DOI] [PubMed] [Google Scholar]
- 2.Bain, G., C. B. Cravatt, C. Loomans, J. Alberola-Ila, S. M. Hedrick, and C. Murre. 2001. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat. Immunol. 2:165-171. [DOI] [PubMed] [Google Scholar]
- 3.Chu, C. C., W. E. Paul, and E. E. Max. 1992. Quantitation of immunoglobulin mu-gamma 1 heavy chain switch region recombination by a digestion-circularization polymerase chain reaction method. Proc. Natl. Acad. Sci. USA 89:6978-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cory, S., and J. M. Adams. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2:647-656. [DOI] [PubMed] [Google Scholar]
- 5.Enders, A., P. Bouillet, H. Puthalakath, Y. Xu, D. M. Tarlinton, and A. Strasser. 2003. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J. Exp. Med. 198:1119-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer, A. M., C. D. Katayama, G. Pages, J. Pouyssegur, and S. M. Hedrick. 2005. The role of erk1 and erk2 in multiple stages of T cell development. Immunity 23:431-443. [DOI] [PubMed] [Google Scholar]
- 7.Fleming, H. E., and C. J. Paige. 2001. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity 15:521-531. [DOI] [PubMed] [Google Scholar]
- 8.Genot, E., and D. A. Cantrell. 2000. Ras regulation and function in lymphocytes. Curr. Opin. Immunol. 12:289-294. [DOI] [PubMed] [Google Scholar]
- 9.Glynne, R., S. Akkaraju, J. I. Healy, J. Rayner, C. C. Goodnow, and D. H. Mack. 2000. How self-tolerance and the immunosuppressive drug FK506 prevent B-cell mitogenesis. Nature 403:672-676. [DOI] [PubMed] [Google Scholar]
- 10.Hasbold, J., L. M. Corcoran, D. M. Tarlinton, S. G. Tangye, and P. D. Hodgkin. 2004. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat. Immunol. 5:55-63. [DOI] [PubMed] [Google Scholar]
- 11.Hatano, N., Y. Mori, M. Oh-hora, A. Kosugi, T. Fujikawa, N. Nakai, H. Niwa, J. Miyazaki, T. Hamaoka, and M. Ogata. 2003. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells 8:847-856. [DOI] [PubMed] [Google Scholar]
- 12.Healy, J. I., R. E. Dolmetsch, L. A. Timmerman, J. G. Cyster, M. L. Thomas, G. R. Crabtree, R. S. Lewis, and C. C. Goodnow. 1997. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity 6:419-428. [DOI] [PubMed] [Google Scholar]
- 13.Hikida, M., S. Johmura, A. Hashimoto, M. Takezaki, and T. Kurosaki. 2003. Coupling between B cell receptor and phospholipase C-gamma2 is essential for mature B cell development. J. Exp. Med. 198:581-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honjo, T., K. Kinoshita, and M. Muramatsu. 2002. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu. Rev. Immunol. 20:165-196. [DOI] [PubMed] [Google Scholar]
- 15.Huntington, N. D., Y. Xu, H. Puthalakath, A. Light, S. N. Willis, A. Strasser, and D. M. Tarlinton. 2006. CD45 links the B cell receptor with cell survival and is required for the persistence of germinal centers. Nat. Immunol. 7:190-198. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda, M., O. Ishida, T. Hinoi, S. Kishida, and A. Kikuchi. 1998. Identification and characterization of a novel protein interacting with Ral-binding protein 1, a putative effector protein of Ral. J. Biol. Chem. 273:814-821. [DOI] [PubMed] [Google Scholar]
- 17.Jacob, J., R. Kassir, and G. Kelsoe. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173:1165-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob, J., and G. Kelsoe. 1992. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J. Exp. Med. 176:679-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz, M. E., and F. McCormick. 1997. Signal transduction from multiple Ras effectors. Curr. Opin. Genet. Dev. 7:75-79. [DOI] [PubMed] [Google Scholar]
- 20.Kolch, W. 2005. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell. Biol. 6:827-837. [DOI] [PubMed] [Google Scholar]
- 21.Ley, R., K. Balmanno, K. Hadfield, C. Weston, and S. J. Cook. 2003. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278:18811-18816. [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y. J., J. Zhang, P. J. Lane, E. Y. Chan, and I. C. MacLennan. 1991. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 21:2951-2962. [DOI] [PubMed] [Google Scholar]
- 23.Luciano, F., A. Jacquel, P. Colosetti, M. Herrant, S. Cagnol, G. Pages, and P. Auberger. 2003. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 22:6785-6793. [DOI] [PubMed] [Google Scholar]
- 24.MacLennan, I. C., A. Gulbranson-Judge, K. M. Toellner, M. Casamayor-Palleja, E. Chan, D. M. Sze, S. A. Luther, and H. A. Orbea. 1997. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol. Rev. 156:53-66. [DOI] [PubMed] [Google Scholar]
- 25.MacLennan, I. C., K. M. Toellner, A. F. Cunningham, K. Serre, D. M. Sze, E. Zuniga, M. C. Cook, and C. G. Vinuesa. 2003. Extrafollicular antibody responses. Immunol. Rev. 194:8-18. [DOI] [PubMed] [Google Scholar]
- 26.Martin, S. W., and C. C. Goodnow. 2002. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat. Immunol. 3:182-188. [DOI] [PubMed] [Google Scholar]
- 27.McHeyzer-Williams, L. J., and M. G. McHeyzer-Williams. 2005. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23:487-513. [DOI] [PubMed] [Google Scholar]
- 28.Muramatsu, M., K. Kinoshita, S. Fagarasan, S. Yamada, Y. Shinkai, and T. Honjo. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102:553-563. [DOI] [PubMed] [Google Scholar]
- 29.Nagaoka, H., Y. Takahashi, R. Hayashi, T. Nakamura, K. Ishii, J. Matsuda, A. Ogura, Y. Shirakata, H. Karasuyama, T. Sudo, S. Nishikawa, T. Tsubata, T. Mizuochi, T. Asano, H. Sakano, and T. Takemori. 2000. Ras mediates effector pathways responsible for pre-B cell survival, which is essential for the developmental progression to the late pre-B cell stage. J. Exp. Med. 192:171-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connor, B. P., V. S. Raman, L. D. Erickson, W. J. Cook, L. K. Weaver, C. Ahonen, L. L. Lin, G. T. Mantchev, R. J. Bram, and R. J. Noelle. 2004. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature 381:751-758. [DOI] [PubMed] [Google Scholar]
- 32.Richards, J. D., S. H. Dave, C. H. Chou, A. A. Mamchak, and A. L. DeFranco. 2001. Inhibition of the MEK/ERK signaling pathway blocks a subset of B cell responses to antigen. J. Immunol. 166:3855-3864. [DOI] [PubMed] [Google Scholar]
- 33.Rickert, R. C., J. Roes, and K. Rajewsky. 1997. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25:1317-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rui, L., C. G. Vinuesa, J. Blasioli, and C. C. Goodnow. 2003. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat. Immunol. 4:594-600. [DOI] [PubMed] [Google Scholar]
- 35.Saba-El-Leil, M. K., F. D. Vella, B. Vernay, L. Voisin, L. Chen, N. Labrecque, S. L. Ang, and S. Meloche. 2003. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 4:964-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro-Shelef, M., and K. Calame. 2005. Regulation of plasma-cell development. Nat. Rev. Immunol. 5:230-242. [DOI] [PubMed] [Google Scholar]
- 37.Shaw, A. C., W. Swat, R. Ferrini, L. Davidson, and F. W. Alt. 1999. Activated Ras signals developmental progression of recombinase-activating gene (RAG)-deficient pro-B lymphocytes. J. Exp. Med. 189:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strasser, A., S. Whittingham, D. L. Vaux, M. L. Bath, J. M. Adams, S. Cory, and A. W. Harris. 1991. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl. Acad. Sci. USA 88:8661-8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sze, D. M. Y., K.-M. Toellner, C. G. de Vinuesa, D. R. Taylor, and I. C. M. MacLennan. 2000. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J. Exp. Med. 192:813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi, Y., A. Inamine, S. Hashimoto, S. Haraguchi, E. Yoshioka, N. Kojima, R. Abe, and T. Takemori. 2005. Novel role of the Ras cascade in memory B cell response. Immunity 23:127-138. [DOI] [PubMed] [Google Scholar]
- 41.Vinuesa, C. G., S. G. Tangye, B. Moser, and C. R. Mackay. 2005. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 5:853-865. [DOI] [PubMed] [Google Scholar]
- 42.Wakabayashi, C., T. Adachi, J. Wienands, and T. Tsubata. 2002. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science 298:2392-2395. [DOI] [PubMed] [Google Scholar]
- 43.Wolthuis, R. M., F. Zwartkruis, T. C. Moen, and J. L. Bos. 1998. Ras-dependent activation of the small GTPase Ral. Curr. Biol. 8:471-474. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi, A., T. Urano, T. Goi, and L. A. Feig. 1997. An Eps homology (EH) domain protein that binds to the Ral-GTPase target, RalBP1. J. Biol. Chem. 272:31230-31234. [DOI] [PubMed] [Google Scholar]