Abstract
Invasive serotype 2 (cps2+) strains of Streptococcus suis cause meningitis in pigs and humans. Four case reports of S. suis meningitis in hunters suggest transmission of S. suis through the butchering of wild boars. Therefore, the objective of this study was to investigate the prevalence of potentially human-pathogenic S. suis strains in wild boars. S. suis was isolated from 92% of all tested tonsils (n = 200) from wild boars. A total of 244 S. suis isolates were genotyped using PCR assays for the detection of serotype-specific genes, the hemolysin gene sly, and the virulence-associated genes mrp and epf. The prevalence of the cps2+ genotype among strains from wild boars was comparable to that of control strains from domestic pig carriers. Ninety-five percent of the cps2+ wild boar strains were positive for mrp, sly, and epf*, the large variant of epf. Interestingly, epf* was significantly more frequently detected in cps2+ strains from wild boars than in those from domestic pigs; epf* is also typically found in European S. suis isolates from humans, including a meningitis isolate from a German hunter. These results suggest that at least 10% of wild boars in Northwestern Germany carry S. suis strains that are potentially virulent in humans. Additional amplified fragment length polymorphism analysis supported this hypothesis, since homogeneous clustering of the epf* mrp+ sly+ cps2+ strains from wild boars with invasive human and porcine strains was observed.
Streptococcus suis is one of the major pathogens in the modern swine industry (13), causing mainly meningitis, septicemia, polyarthritis, endocarditis, and pneumonia. A number of biotic and abiotic factors are thought to play an important role in the epidemiologies of these diseases. Other pathogens of the porcine respiratory tract, such as the porcine reproductive and respiratory virus, may increase the host's susceptibility (9). Abiotic factors that may predispose piglets to S. suis infection are, for example, corrosive gases and weaning, transport, and crowding of piglets.
S. suis has also been identified as a causative agent of meningitis, septicemia, arthritis, endocarditis, hearing loss, and ocular diseases in humans (2, 16, 18, 19, 21, 27, 33, 35). This zoonosis appears to be rather common in Hong Kong and Thailand (8, 21, 35) but less common in Central Europe (2) and almost unknown in North America (11). Recently, in an unusual outbreak of 215 cases of S. suis diseases in humans in Sichuan, People's Republic of China, 38 deaths occurred (43). The high lethality was related to a toxic shock-like syndrome, which was yet unknown for S. suis infections, except in one case (34). Based on the histories of numerous human S. suis cases including the outbreak in Sichuan, the processing of pork is considered to be a major risk factor for this zoonosis (2, 22, 43). The histories of previously described S. suis diseases suggest that injured human skin is a major entry site for the pathogen (2, 43).
S. suis is a heterogeneous species that can be divided into at least 33 serotypes (13, 17). Worldwide, capsular serotype 2 is the most prevalent one among invasive porcine and human isolates (23, 29, 42). Smith et al. previously demonstrated protection against phagocytosis through the expression of the capsule in serotype 2 strains (30). However, it is well known that strains within serotype 2 may differ substantially in virulence (31, 38, 42). The muramidase-released protein (MRP) (mrp gene) and the extracellular factor (EF) (epf gene) have been proven to be suitable markers for virulence of serotype 2 strains (38, 42). However, their function is still unknown, and mutational inactivation of both genes did not result in an attenuation of virulence (32). Different size variants of MRP and EF can be distinguished. In serotype 2 strains, MRP and EF, with sizes of 136 and 110 kDa, respectively, are expressed by highly virulent strains (MRP+ and EF+) that have been shown to induce meningitis and septicemia in experimentally infected piglets (32). Serotype 2 strains that express a large variant of EF (EF*) are considered to be less virulent in piglets (31). However, European human S. suis isolates frequently belong to this genotype (mrp+ epf* cps2 strains) (31). Similar to EF, MRP is also highly variable. In addition to serotype 2 strains, serotype 9 strains, which carry a large version of the mrp gene (mrp*), are epidemiologically important for invasive porcine, but not human, S. suis infections in Europe (29, 42).
A number of other putative virulence factors in S. suis have been described. These include suilysin, a hemolysin that is expressed by most European and Asian S. suis isolates but is very rarely expressed by those from North America (12, 20, 28). Suilysin has cytolytic functions, may be involved in the invasion of eucaryotic cells, and can affect complement-mediated opsonization (6). A putative S. suis survival factor recently identified by our group is arginine deiminase (AdiS), encoded by the gene arcA (3, 40). Almost all S. suis strains isolated from pigs carry this gene (23).
In addition to domesticated pigs, wild boars (both Sus scrofa) have been proposed to be a reservoir for virulent S. suis strains. This hypothesis is based on cases of S. suis diseases in hunters after butchering wild boars (5, 14, 15, 27). In Germany, wild boars have become very abundant, and during the last few years, more than 400,000 animals were shot annually (www.jagd-online.de). The butchering of these animals is very often performed under limited light conditions and without gloves, thus increasing the risk of infection through skin wounds. However, knowledge of the epidemiological distribution of S. suis in wild boars is very limited.
In this study, we addressed the hypothesis that wild boars are carriers of putative human-pathogenic S. suis strains. We assumed that S. suis strains with the same, or similar, virulence-associated gene profiles and DNA fingerprints as those isolated from humans should be detectable in wild boars. Therefore, isolates from wild boars in Northwestern Germany were characterized by virulence-associated gene and amplified fragment length polymorphism (AFLP) typing. As domestic pigs are known to be a reservoir of virulent S. suis strains, we included not only isolates from humans but also isolates from domestic pigs for comparison. Our results suggest that wild boars in Northwestern Germany frequently carry putative zoonotic S. suis strains.
MATERIALS AND METHODS
Samples and bacterial strains.
Tonsils from 200 wild boars shot in Northwestern Germany were collected and processed for bacterial culture within 24 h after the death of the animal. Samples were collected in 12 different regions. No region contained more than 14% of the samples used. As two highways intersect the area of sample collection, it is very likely that at least four populations of wild boars with little exchange are represented. Also included in this study were 20 wild boars shot in the Saupark Springe game park, which has been surrounded by a wall since 1839. This park has a size of 16 km2 and harbors a large population of wild boars (www.saupark-springe.de).
More than one isolate per animal was included only if it showed a distinct multiplex PCR (MP-PCR) genotype with regard to the first isolate(s). For comparison, S. suis isolates from tonsils of pigs (n = 92) that were either healthy or dissected for reasons other than S. suis infection in Northwestern Germany were investigated as well (domestic carrier group). In both groups, 70 to 80% of the animals were between 4 weeks and 8 months of age. Both sexes were represented. Samples were streaked onto Columbia and Streptococcus/Staphylococcus selective agar plates (Oxoid, Wesel, Germany) with 6% sheep blood. From each sample, up to four subcultures (depending on the different colony morphologies) of alpha-hemolytic streptococci were used for the preparation of chromosomal DNA. Isolates were further cultivated on Columbia agar with sheep blood and in Todd-Hewitt broth (Oxoid).
Preparation of chromosomal DNA.
Chromosomal DNA was prepared according to standard procedures (39).
PCR.
A previously described MP-PCR was used to identify and differentiate S. suis isolates in a single-step procedure (29). The identification of S. suis was based on the detection of a specific gdh amplification product. The MP-PCR allowed the identification of four capsule types (types 1, 2, 7, and 9) through oligonucleotide primers targeting the cps locus and the detection of four virulence-associated genes (mrp, epf, sly, and arcA).
Differentiation of mrp and epf variants was done by monoplex PCR assays as described previously (29).
For comparison, 39 selected tonsils from wild boars in 10 of the 12 different regions were also investigated using a multiplex PCR recently described by Marois et al. (24). Template DNA for this MP-PCR (100 ng per reaction) was prepared from a 10-ml Todd-Hewitt broth culture with Streptococci Selective Supplement (Oxoid) inoculated with the tonsil specimen.
AFLP.
Single-enzyme (HindIII) AFLP typing was done with 70 selected strains from wild boars, 8 porcine reference strains, and 5 human strains. Selection was based on the results of virulence-associated gene profiling. All except two cps2+ isolates were included (the two cps2+ strains not investigated had exactly the same virulence-associated gene profile and were from the same region as at least three other strains that were included). Each of the 22 different genotypes (regarding cps1/2/7/9, sly, mrp, epf, and arcA) identified among S. suis isolates from wild boars in this study was represented. Additional strains were selected randomly. AFLP was performed as described previously for Helicobacter pylori (10), with the following modifications. Briefly, 1 μg of digested DNA was used in the ligation reaction mixture containing 30 μM of annealed adapter ADH1/ADH2, 1× T4 DNA ligase buffer, and 1 U T4 DNA ligase (both from Promega, Mannheim, Germany) in a final volume of 20 μl. Subsequently, PCR amplification products were generated with 1 μM primer HI-G (10) and separated by 2.5% (wt/vol) agarose gel electrophoresis. AFLP patterns were analyzed using BioNumerics software 4.0 (AppliedMath, Sint-Martens Latem, Belgium). The pairwise comparison of band patterns was performed using the Pearson product-moment correlation coefficient, and the dendrogram was calculated by the unweighted-pair group method analysis using average linkages.
Sequencing.
The epf3004 amplification product of strain W50.2 generated with the epf-specific primer pair was cloned and sequenced as described previously (29). The obtained epf3004 sequence and the previously published sequence of epf1890 (GenBank accession no. A24024) were used for additional primer design to generate overlapping PCR amplification products of epf3004 of strain W50.2. These amplification products were cloned and sequenced by primer walking.
Statistical evaluation.
Fisher's two-sided exact test was used to compare the prevalences of strains with a specific gene (e.g., sly+) or genotype (e.g., sly+ mrp+ epf* cps2) in the two different groups. Differences were estimated as significant when probabilities (P) were lower than 0.05.
Nucleotide sequence accession number.
The full-length sequence of epf3004 has been deposited in the GenBank database under accession no. DQ372915.
RESULTS
Prevalence of S. suis and detection of serotype-specific genes.
S. suis was isolated from 92% of all 200 tested tonsils from wild boars based on the generation of the gdh-specific PCR amplification product from alpha-hemolytic streptococci (26). More than one genotype of S. suis was isolated from 61 (30.5%) wild boars. Among the total 244 S. suis isolates, 22 different genotypes could be differentiated using previously described (29) MP-PCR and mrp and epf variant PCR, respectively. Eighteen different genotypes were detected in the control group of 92 S. suis isolates from domestic pigs. Three of the four investigated capsular genotypes were detected in isolates from wild boars, namely, cps2, cps7, and cps9 but not cps1 (Table 1). The prevalence of cps2 strains was similar in wild boars (11%) and domestic pigs (14%). In contrast, cps9 strains were significantly (P < 0.0001) more often detected in wild boars (22%) than in domestic pigs (2%). More than 70% of the genotyped S. suis isolates from both groups (wild boars and domestic pigs) did not belong to the genotypes cps1, cps2, cps7, or cps9.
TABLE 1.
Prevalence of different S. suis genotypes among wild boars and domestic pigs in Northwestern Germany
| Genotype |
S. suis isolates from:
|
|||
|---|---|---|---|---|
| Wild boars
|
Domestic pigs—prevalence (no. of animals with genotype/total no. of animals)a | |||
| Prevalence (no. of animals with genotype/total no. of animals)a | No. of regions with positive animal/total no. of regions investigatedb | AFLP cluster A (no. of strains with cluster A/total no. of strains investigated)c | ||
| cps1 | 0/200 | 0/12 | 0/0 | 1/92 |
| sly+mrp+epf* cps1 | 0/200 | 0/12 | 0/0 | 1/92 |
| cps2 | 22/200 | 4/12 | 19/20 | 13/92 |
| sly+mrp+epf+cps2 | 0/200 | 0/12 | 0/0 | 5/92 |
| sly negative mrp+epf negative cps2 | 1/200 | 1/12 | 0/1 | 5/92 |
| sly+mrp+epf negative cps2 | 0/200 | 0/12 | 0/0 | 1/92 |
| mrp+epf* sly+cps2d | 21/200e | 4/12 | 19/19 | 2/92e |
| cps7 | 4/200 | 3/12 | 0/4 | 3/92 |
| sly+mrp** cps7 | 1/200 | 1/12 | 0/1 | 0/92 |
| sly+mrp negative cps7 | 3/200 | 2/12 | 0/3 | 3/92 |
| cps9 | 44/200e | 10/12 | 1/22 | 2/92e |
| sly+mrp+cps9 | 1/200 | 1/12 | 0/1 | 0/92 |
| sly+mrp* cps9 | 1/200 | 1/12 | 0/1 | 0/92 |
| sly+mrp** cps9 | 1/200 | 1/12 | 0/1 | 0/92 |
| sly+mrp*** cps9 | 2/200 | 2/12 | 1/2 | 1/92 |
| sly+mrp negative cps9 | 8/200 | 6/12 | 0/4 | 0/92 |
| sly negative mrp negative cps9 | 31/200 | 10/12 | 0/13 | 1/92 |
| Nontypeablef | 174/200 | 12/12 | 3/24 | 73/92 |
Number of investigated animals from which this genotype was isolated.
Number of investigated regions with at least one wild boar from which this genotype was isolated.
Number of strains investigated by AFLP with this genotype that were assigned to cluster A. Note that cluster A is defined by a 68% similarity cutoff and contains all five investigated human European isolates.
All five investigated human European isolates showed this genotype.
Significant difference between wild boars and domestic pigs.
Isolates were not cps1, cps2, cps7, or cps9.
Thirty-nine tonsils from wild boars from 10 regions were also investigated for cps2 strains by an approach based on the MP-PCR described previously by Marois et al. (24). This approach avoids the isolation of strains. In 10 of the 39 samples, a cps2-specific fragment was generated. However, correlation with the results of genotyping as described above was rather low. In six samples, cps2 was detected by both methods, four samples were positive only by the MP-PCR described previously by Marois et al. (24), and four samples did not show a cps2 amplification product in the latter, although a cps2+ strain was isolated and detected with the MP-PCR described above by Silva et al. (29).
The prevalences of cps2+ wild boars differed among the 13 regions investigated. One region had a very high carrier rate of cps2+ strains (58%). No cps2 strain was detectable with either of the two approaches in wild boars from 6 of the 13 regions.
Prevalence of arcA and sly.
Each of the four additional virulence-associated genes investigated (arcA, sly, mrp, and epf) was present in isolates from wild boars (Fig. 1 and Table 1). All isolates from wild and domestic pigs were positive for arcA. In general, the gene encoding suilysin, sly, was significantly less frequently detected in isolates from wild boars (39% positive) than in isolates from domestic pigs (66% positive; P < 0.0001). However, all except 1 of the 22 cps2 strains from wild boars were positive for sly (Table 1).
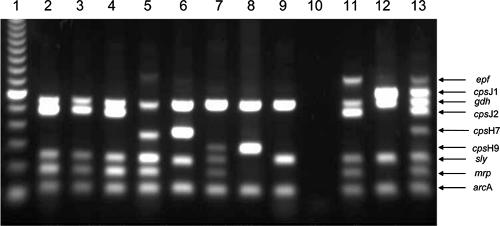
FIG. 1.
Representative MP-PCR of S. suis strains. Lanes: 1, 100-bp ladder; 2, meningitis isolate from a German hunter (strain 199) (27); 3 to 9, isolates from tonsils of wild boars (W183.1, W168.1, W102.2, W31.3, W162.1, W151.2, and W184.1); 10, water; 11, reference strain P1/7; 12, serotype 1 reference strain DSM 9683; 13, mixture of different reference strains (29).
Prevalence and variability of mrp.
The gene encoding the muramidase-released protein, mrp (including all variants), was significantly less frequently detected in isolates from wild boars (18%) than in isolates from domestic pigs (43%; P < 0.0001). Different size variants of MRP have been described previously (31, 42). PCR assays with primers targeting sequences flanking the variable region of the mrp gene were used to distinguish these size variants. Thus, MP-PCR was used in combination with the specific monoplex PCR for discrimination of mrp variants (27). All cps2+ isolates from wild boars and domestic pigs investigated were positive for the 136-kDa-protein-encoding mrp gene (mrp+) (Table 1). S. suis isolates from wild boars other than cps2 isolates showed size variations of mrp similar to those of isolates from domestic pigs (Fig. 2A). In cps9+ strains from wild boars, four different mrp variants were distinguishable. However, only one wild boar cps9 isolate was positive for the mrp* variant, which is frequently detected in invasive serotype 9 isolates from domestic pigs, as represented by strain A3286/94 (1, 29, 42).
FIG. 2.
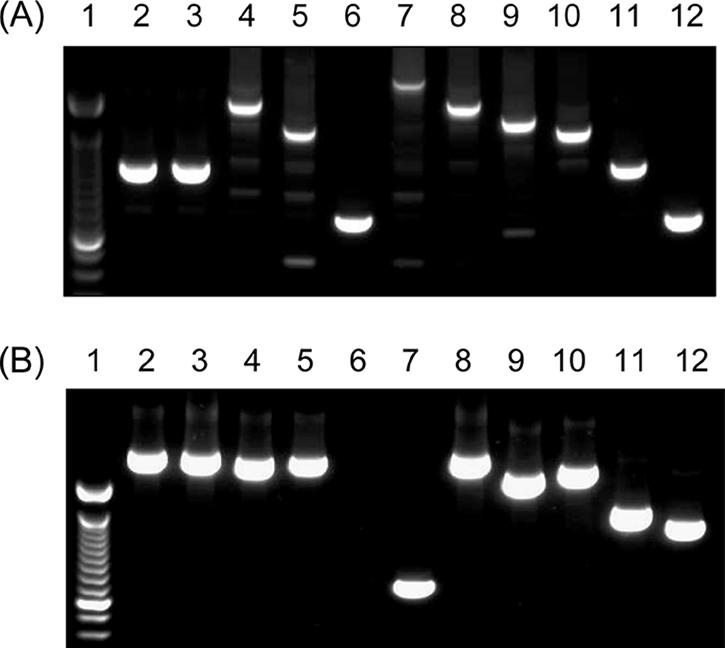
Representative mrp (A) and epf (B) PCR of S. suis strains for differentiation of size variants. (A) Lane 1, 100-bp ladder; lane 2, meningitis isolate from a German hunter (strain 199) (27); lanes 3 to 6, isolates from tonsils of wild boars (W183.1, W123.3, W131.1, and W108.2); lanes 7 to 12, mrp reference strains (V7353/1, 90-2741-7, A5373/4, A3286/94, D282, and A5140/3/96) (29). (B) Lane 1, 100-bp ladder; lane 2, strain 199 (see A); lane 3, isolate from a butcher (MAC 724); lanes 4 and 5, cps2+ isolates from tonsils of wild boars (W50.2 and W50.3); lane 6, negative control (A5683/93); lane 7, epf+ control strain (P1/7); lanes 8 to 12, epf* reference strains (lane 8, 1890; lane 9, 2840; lane 10, 3921; lane 11, 3988; lane 12, 3995) (29, 31).
Analysis of the epf genotype in cps2 strains isolated from wild boars.
None of the cps2 isolates from wild boars generated the epf amplification product specific for the gene (epf+) coding for the 110-kDa EF protein. This important virulence marker was also rarely found in isolates from domestic pigs (Table 1). The cps2+ strains were screened with epf variant PCR (27) to detect large variants of epf, generally named epf* (29, 31). Sixteen strains from wild boars generated an amplification product that was different in size from those of the products of all known epf variants (Fig. 2B, lane 4). The particular gene, called epf3004, of strain W50.2 was sequenced completely and was found to be very similar to epf1890 (96% identity). The only differences were one single nucleotide exchange and the deletion of repeat 6 in epf1890 (nucleotides 4021 to 4248). Ninety-five percent of serotype 2 wild boar strains, which were shot in four different regions, were positive for one of these two very similar size variants of epf* (epf1890 and epf3004) (Fig. 2). Importantly, all five European human isolates investigated, including the meningitis isolate from a German hunter (Fig. 2B, lane 2), also showed the genotype sly+ mrp+ epf* (and in particular epf1890) cps2+. The prevalence of sly+ mrp+ epf* cps2+ strains was significantly higher in wild boars than in domestic pigs (Table 1).
AFLP typing.
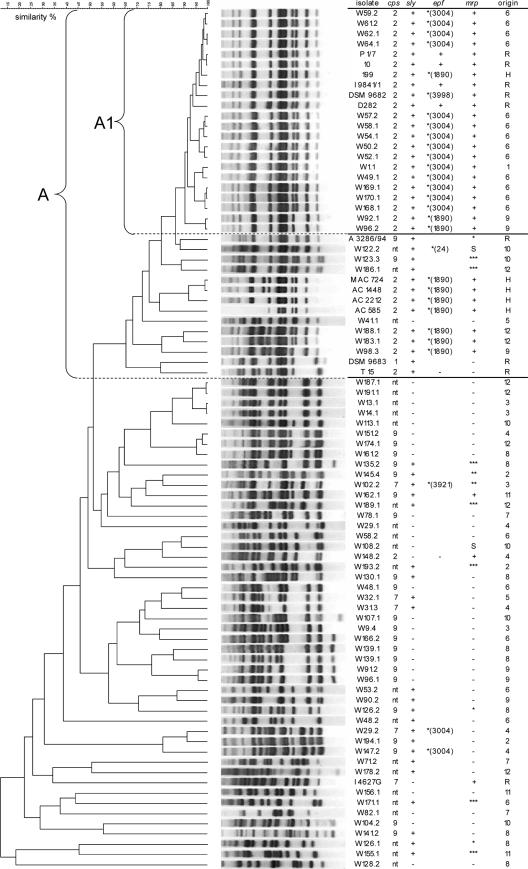
In a preliminary study, we developed an AFLP typing procedure for S. suis and identified a homogeneous cluster (A1) associated with sly+ mrp+ epf+ (or epf*) cps2+ strains of porcine and human origin with an invasive clinical background (26a). In the present study, 70 wild boar isolates, 5 European human isolates, and 9 porcine reference strains were typed by this AFLP approach. Of the wild boar strains, 20 cps2+, all 4 cps7+, 22 cps9+, and 24 cps-nontypeable strains representing all regions were included. As shown in Fig. 3, 16 (80%) of the AFLP-typed cps2+ wild boar isolates clustered at a linkage level of 90% within cluster A1, which additionally included only the hunter isolate, strain 199 (27), and the 5 highly virulent sly+ mrp+ epf+ serotype 2 reference strains, 10 (30, 32), P1/7 (20), I9841/1 (1, 40), DSM 9682, and D282 (38). Closely associated with this cluster (≥68% similarity, cluster A) were seven additional wild boar strains (three cps2+, one cps9+, and three cps nontypeable), four European human strains (strains 122, 126, 124, and 127), and three porcine reference strains (mrp* serotype 9 reference strain A3286/94 [1, 29], serotype 1 strain DSM 9683, and serotype 2 strain T15). All other wild boar strains were very heterogeneous (Fig. 3). Some S. suis strains, such as strains w48.2 and w128.2, showed a similarity to any other strain of less than 50%. Neither of the investigated cps9+ and cps7+ wild boar strains formed an AFLP cluster comparable in similarity and number of strains to the described cluster A1 (A). Interestingly, there was a very high diversity among cps9 strains. Only one of the 44 cps9 strains from wild boars (w123.3) generated a pattern with a similarity level of more than 60% compared to the pattern of invasive cps9 reference strain A3286/94 (1).
FIG. 3.
AFLP dendrogram of 70 S. suis isolates from wild boars (indicated by “W” under isolate), 5 human strains (indicated by “H” under origin), and 9 porcine reference strains (indicated by “R” under origin). The pairwise comparison of band patterns was performed using the Pearson product-moment correlation coefficient. The numbers in the parentheses specifying the epf* variant are equivalent to the names of the reference strains with the same-sized epf* (29, 31), except for the variant found only in wild boars (epf3004). The “+” under epf and mrp refers to the variants that encode the 110-kDa EF and 136-kDa MRP, respectively. The numbers under origin refer to the 12 regions of sample collection in Northwestern Germany.
DISCUSSION
S. suis is a zoonotic pathogen that has received only limited attention with respect to epidemiology in humans, except for the recent outbreak in Sichuan, People's Republic of China, in 2005 (11, 34, 43). The current knowledge about the zoonotic aspect of S. suis is based on a few larger case series (2, 21, 33, 43), numerous single case reports, and few epidemiological studies of pigs that included human isolates (4, 7, 23, 31). Altogether, more than 100 human S. suis isolates have been serotyped (2, 4, 7, 23, 27, 31, 33). The results strongly suggest that serotype 2 (cps2) and probably also serotype 1 (cps1) strains have a higher zoonotic potential than other S. suis serotypes. This is in accordance with the high prevalence of serotype 2 strains among invasive porcine isolates (42). However, the zoonotic potential of other (non-serotype 2) invasive porcine S. suis pathotypes is unknown. In particular, serotype 9 strains have a high prevalence among S. suis isolates from diseased pigs with meningitis and other invasive diseases in Europe (42). However, to our knowledge, no human isolate belonging to serotype 9 has been described so far.
In this study, we demonstrated by two different approaches that tonsils of wild boars in Northwestern Germany are frequently colonized with cps2+ strains (more than 10%). We propose that the majority, if not all, of the cps2+ strains from wild boars detected in this study are virulent and putative zoonotic agents. Firstly, all except one of these strains were also positive for the virulence-associated factors sly and mrp. In addition, 96% of these strains carried a large variant of the virulence-associated factor epf (epf*). These large variants are found in serotype 2 strains of moderate virulence in piglets but are also typically detected in human European isolates (25, 29, 31). In agreement with this, the invasive human European isolates genotyped in this study were all positive for the particular large variant epf1890. The same variant and a closely related variant, epf3004, were detected among the wild boar isolates investigated in this study. The putative zoonotic potential of these cps2+ wild boar strains (with the exception of strain w148.2) is supported by our finding that 6 of 19 strains were completely, and the remaining strains were nearly, indistinguishable from a meningitis isolate from a hunter who was infected with S. suis after butchering a wild boar in Northern Germany (27). Furthermore, the 19 cps2+ wild boar isolates belonged to AFLP cluster A, which showed a high overall similarity level (>60%) and, in addition, harbored only highly virulent porcine and human S. suis strains. Homogeneous clustering of invasive cps2 (and cps1) S. suis strains has previously been observed using the same typing method (26a) and other typing methods (23).
AFLP allowed the differentiation of two populations of cps2 strains that showed a similarity of less than 45% to each other. The cluster of invasive cps2 strains is represented by virulent mrp+ epf+ sly+ cps2+ reference strains, such as strains P1/7, 10, and D282 (26a). These strains clustered with a very high similarity together with the cps2+ wild boar isolates, except for strain W148.2. Thus, these cps2+ wild boar isolates can be considered to belong to cluster A and not to cluster C as defined previously by Rehm et al. (26a). Accordingly, the cps2 strains of cluster C were all negative for sly and epf. Strains of AFLP cluster A most probably belong to the multilocus sequence type 1 complex described previously by King et al. (23), since strains investigated with both typing methods, especially invasive cps2 strains, formed similar clusters with both methods (26a). Interestingly, the vast majority (87%) of the human isolates investigated with multilocus sequence typing also belonged to the sequence type 1 complex (23).
Despite the large number (n = 244) of genotyped S. suis isolates from wild boars, sly+ mrp+ epf+ (coding for the 110-kDa EF protein) cps2+ genotypes were not detected in this group, in contrast to the smaller control group of isolates from domestic pigs (Table 1) and the high prevalence of this genotype among invasive porcine isolates (29, 42). In addition, only one wild boar isolate belonged to the genotype mrp* sly+ cps9, which is also very common among clinical isolates from pigs in Europe (29, 42). In domestic pigs, these two important invasive S. suis genotypes are detectable not only in specimens from diseased pigs but also in tonsillar specimens from healthy carriers, which are very frequent in swine herds with S. suis problems (41). Based on our results, it may be speculated that factors associated with modern swine production, such as early weaning, high concentrations of animals, and corrosive gases, led to the selection of highly virulent S. suis strains that are less frequent or even absent in wild boars. The high virulence of these strains might, however, be specific for domestic pigs and less adapted to the human host. The aspect of host-specific virulence of S. suis strains has been demonstrated in experimental infections of mice and pigs (36, 37). In contrast to sly+ mrp+ epf+ cps2 strains, sly+ mrp+ epf* cps2 strains might express virulence factors that are less host specific. Reduced host specificity of virulence factors might be disadvantageous for survival in modern swine production but advantageous for the zoonotic potential of these strains. Though epf is not an essential virulence factor for S. suis serotype 2 strains (32), it is probably associated with other factors that play a more crucial role in determining virulence and host specificity in S. suis strains. Their identification and functional characterization are an important goal of future studies of the pathogenesis and epidemiology of S. suis in pigs and humans.
Acknowledgments
The S. suis isolate from the hunter (strain 199) and the reference strains were kindly provided by I. Sobottka (Universitätsklinikum Hamburg-Eppendorf, Germany) and H. Smith (DLO-Lelystad, The Netherlands), respectively. We are also grateful to numerous forestry offices in Lower Saxony for their help with sample collection.
Luciana M. G. Silva was financially supported by CNPq-Brasilia/Brazil. This study was supported by a grant from the Niedersächsisches Ministerium für den ländlichen Raum, Ernährung, Landwirtschaft, und Verbraucherschutz and by the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany (SFB587).
Footnotes
Published ahead of print on 3 November 2006.
REFERENCES
- 1.Allgaier, A., R. Goethe, H. J. Wisselink, H. E. Smith, and P. Valentin-Weigand. 2001. Relatedness of Streptococcus suis isolates of various serotypes and clinical backgrounds as evaluated by macrorestriction analysis and expression of potential virulence traits. J. Clin. Microbiol. 39:445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arends, J. P., and H. C. Zanen. 1988. Meningitis caused by Streptococcus suis in humans. Rev. Infect. Dis. 10:131-137. [DOI] [PubMed] [Google Scholar]
- 3.Benga, L., R. Goethe, M. Rohde, and P. Valentin-Weigand. 2004. Non-encapsulated strains reveal novel insights in invasion and survival of Streptococcus suis in epithelial cells. Cell. Microbiol. 6:867-881. [DOI] [PubMed] [Google Scholar]
- 4.Berthelot-Herault, F., C. Marois, M. Gottschalk, and M. Kobisch. 2002. Genetic diversity of Streptococcus suis strains isolated from pigs and humans as revealed by pulsed-field gel electrophoresis. J. Clin. Microbiol. 40:615-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonmarchand, G., P. Massari, G. Humbert, J. Leroy, A. Morel, J. F. Lemeland, and P. Vannier. 1985. Group-R streptococci—wild boars as a 2nd reservoir. Scand. J. Infect. Dis. 17:121-122. [DOI] [PubMed] [Google Scholar]
- 6.Chabot-Roy, G., P. Willson, M. Segura, S. Lacouture, and M. Gottschalk. 2006. Phagocytosis and killing of Streptococcus suis by porcine neutrophils. Microb. Pathog. 41:21-32. [DOI] [PubMed] [Google Scholar]
- 7.Chatellier, S., M. Gottschalk, R. Higgins, R. Brousseau, and J. Harel. 1999. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J. Clin. Microbiol. 37:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chau, P. Y., C. Y. Huang, and R. Kay. 1983. Streptococcus suis meningitis. Med. J. Aust. 1:414-417. [PubMed] [Google Scholar]
- 9.Galina, L., C. Pijoan, M. Sitjar, W. T. Christianson, K. Rossow, and J. E. Collins. 1994. Interaction between Streptococcus suis serotype 2 and porcine reproductive and respiratory syndrome virus in specific pathogen free piglets. Vet. Rec. 134:60-64. [DOI] [PubMed] [Google Scholar]
- 10.Gibson, J. R., E. Slater, J. Xerry, D. S. Tompkins, and R. J. Owen. 1998. Use of an amplified-fragment length polymorphism technique to fingerprint and differentiate isolates of Helicobacter pylori. J. Clin. Microbiol. 36:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschalk, M. 2004. Porcine Streptococcus suis strains as potential sources of infections in humans: an underdiagnosed problem in North America? J. Swine Health Prod. 12:197-199. [Google Scholar]
- 12.Gottschalk, M., A. Lebrun, H. Wisselink, J. D. Dubreuil, H. Smith, and U. Vecht. 1998. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can. J. Vet. Res. 62:75-79. [PMC free article] [PubMed] [Google Scholar]
- 13.Gottschalk, M., and M. Segura. 2000. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 76:259-272. [DOI] [PubMed] [Google Scholar]
- 14.Grebe, T., D. Bergenthal, A. M. Fahr, and H. W. Scheja. 1997. Meningitis caused by Streptococcus suis type 2 in an adult. Deutsch Med. Wochenschr. 122:1244-1247. [DOI] [PubMed] [Google Scholar]
- 15.Halaby, T., E. Hoitsma, R. Hupperts, L. Spanjaard, M. Luirink, and J. Jacobs. 2000. Streptococcus suis meningitis, a poacher's risk. Eur. J. Clin. Microbiol. Infect. Dis. 19:943-945. [DOI] [PubMed] [Google Scholar]
- 16.Heidt, M. C., W. Mohamed, T. Hain, P. R. Vogt, T. Chakraborty, and E. Domann. 2005. Human infective endocarditis caused by Streptococcus suis serotype 2. J. Clin. Microbiol. 43:4898-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill, J. E., M. Gottschalk, R. Brousseau, J. Harel, S. M. Hemmingsen, and S. H. Goh. 2005. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet. Microbiol. 107:63-69. [DOI] [PubMed] [Google Scholar]
- 18.Huang, Y. T., L. J. Teng, S. W. Ho, and P. R. Hsueh. 2005. Streptococcus suis infection. J. Microbiol. Immunol. Infect. 38:306-313. [PubMed] [Google Scholar]
- 19.Hui, A. C., K. C. Ng, P. Y. Tong, V. Mok, K. M. Chow, A. Wu, and L. K. Wong. 2005. Bacterial meningitis in Hong Kong: 10-years' experience. Clin. Neurol. Neurosurg. 107:366-370. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs, A. A. C., P. L. W. Loeffen, A. J. G. van den Berg, and P. K. Storm. 1994. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect. Immun. 62:1742-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kay, R., A. F. Cheng, and C. Y. Tse. 1995. Streptococcus suis infection in Hong-Kong. QJM 88:39-47. [PubMed] [Google Scholar]
- 22.Kay, R., A. F. Cheng, and C. Y. Tse. 1995. Streptococcus suis meningitis in pork handlers. Neurology 45:A370. [Google Scholar]
- 23.King, S. J., J. A. Leigh, P. J. Heath, I. Luque, C. Tarradas, C. G. Dowson, and A. M. Whatmore. 2002. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: identification of virulent clones and potential capsular serotype exchange. J. Clin. Microbiol. 40:3671-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marois, C., S. Bougeard, M. Gottschalk, and A. Kobisch. 2004. Multiplex PCR assay for detection of Streptococcus suis species and serotypes 2 and 1/2 in tonsils of live and dead pigs. J. Clin. Microbiol. 42:3169-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez, G., A. F. P. de Castro, K. J. R. Pagnani, G. Nakazato, W. D. da Silveira, and M. Gottschalk. 2003. Clonal distribution of an atypical MRP+, EF*, and suilysin(+) phenotype of virulent Streptococcus suis serotype 2 strains in Brazil. Can. J. Vet. Res. 67:52-55. [PMC free article] [PubMed] [Google Scholar]
- 26.Okwumabua, O., M. O'Connor, and E. Shull. 2003. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiol. Lett. 218:79-84. [DOI] [PubMed] [Google Scholar]
- 26a.Rehm, T., C. G. Baums, B. Strommenger, M. Beyerbach, P. Valentin-Weigand, and R. Goethe. 2007. Amplified fragment length polymorphism of Streptococcus suis strains correlates with their profiles of virulence-associated genes and clinical background. J. Med. Microbiol. 56:102-109. [DOI] [PubMed] [Google Scholar]
- 27.Rosenkranz, M., H. A. Elsner, H. J. Sturenburg, C. Weiller, J. Rother, and I. Sobottka. 2003. Streptococcus suis meningitis and septicemia contracted from a wild boar in Germany. J. Neurol. 250:869-870. [DOI] [PubMed] [Google Scholar]
- 28.Segers, R. P. A. M., T. Kenter, L. A. M. de Haan, and A. A. C. Jacobs. 1998. Characterisation of the gene encoding suilysin from Streptococcus suis and expression in field strains. FEMS Microbiol. Lett. 167:255-261. [DOI] [PubMed] [Google Scholar]
- 29.Silva, L., C. G. Baums, T. Rehm, H. Wisselink, R. Goethe, and P. Valentin-Weigand. 2006. Virulence-associated gene profiling of Streptococcus suis isolates by PCR. Vet. Microbiol. 155:117-127. [DOI] [PubMed] [Google Scholar]
- 30.Smith, H. E., M. Damman, J. van der Velde, F. Wagenaar, H. J. Wisselink, N. Stockhofe-Zurwieden, and M. A. Smits. 1999. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 67:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, H. E., F. H. Reek, U. Vecht, A. L. J. Gielkens, and M. A. Smits. 1993. Repeats in an extracellular protein of weakly pathogenic strains of Streptococcus suis type 2 are absent in pathogenic strains. Infect. Immun. 61:3318-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, H. E., U. Vecht, H. J. Wisselink, N. Stockhofe-Zurwieden, Y. Biermann, and M. A. Smits. 1996. Mutants of Streptococcus suis types 1 and 2 impaired in expression of muramidase-released protein and extracellular protein induce disease in newborn germfree pigs. Infect. Immun. 64:4409-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suankratay, C., P. Intalapaporn, P. Nunthapisud, K. Arunyingmongkol, and H. Wilde. 2004. Streptococcus suis meningitis in Thailand. Southeast Asian J. Trop. Med. Public Health 35:868-876. [PubMed] [Google Scholar]
- 34.Tang, J., C. Wang, Y. Feng, W. Yang, H. Song, Z. Chen, H. Yu, X. Pan, X. Zhou, H. Wang, B. Wu, H. Wang, H. Zhao, Y. Lin, J. Yue, Z. Wu, X. He, F. Gao, A. H. Khan, J. Wang, G. P. Zhao, Y. Wang, X. Wang, Z. Chen, and G. F. Gao. 2006. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. 3:e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trottier, S., R. Higgins, G. Brochu, and M. Gottschalk. 1991. A case of human endocarditis due to Streptococcus suis in North America. Rev. Infect. Dis. 13:1251-1252. [DOI] [PubMed] [Google Scholar]
- 36.Vecht, U., N. Stockhofe-Zurwieden, B. J. Tetenburg, H. J. Wisselink, and H. E. Smith. 1997. Virulence of Streptococcus suis type 2 for mice and pigs appeared host-specific. Vet. Microbiol. 58:53-60. [DOI] [PubMed] [Google Scholar]
- 37.Vecht, U., N. Stockhofe-Zurwieden, B. J. Tetenburg, H. J. Wisselink, and H. E. Smith. 1997. Murine and pig models of Streptococcus suis type 2 infections are incompatible. Adv. Exp. Med. Biol. 418:827-829. [DOI] [PubMed] [Google Scholar]
- 38.Vecht, U., H. J. Wisselink, M. L. Jellema, and H. E. Smith. 1991. Identification of 2 proteins associated with virulence of Streptococcus suis type 2. Infect. Immun. 59:3156-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson, K. 2000. Preparation and analysis of DNA, p. 2.4.1.-2.4.5. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., Hoboken, NJ.
- 40.Winterhoff, N., R. Goethe, P. Gruening, M. Rohde, H. Kalisz, H. E. Smith, and P. Valentin-Weigand. 2002. Identification and characterization of two temperature-induced surface-associated proteins of Streptococcus suis with high homologies to members of the arginine deiminase system of Streptococcus pyogenes. J. Bacteriol. 184:6768-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisselink, H. J., F. H. Reek, U. Vecht, N. Stockhofe-Zurwieden, M. A. Smits, and H. E. Smith. 1999. Detection of virulent strains of Streptococcus suis type 2 and highly virulent strains of Streptococcus suis type 1 in tonsillar specimens of pigs by PCR. Vet. Microbiol. 67:143-157. [DOI] [PubMed] [Google Scholar]
- 42.Wisselink, H. J., H. E. Smith, N. Stockhofe-Zurwieden, K. Peperkamp, and U. Vecht. 2000. Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis strains isolated from diseased pigs in seven European countries. Vet. Microbiol. 74:237-248. [DOI] [PubMed] [Google Scholar]
- 43.Yu, H., H. Jing, Z. Chen, H. Zheng, X. Zhu, H. Wang, S. Wang, L. Liu, R. Zu, L. Luo, N. Xiang, H. Liu, X. Liu, Y. Shu, S. S. Lee, S. K. Chuang, Y. Wang, J. Xu, and W. Yang. 2006. Human Streptococcus suis outbreak, Sichuan, China. Emerg. Infect. Dis. 12:914-920. [DOI] [PMC free article] [PubMed] [Google Scholar]