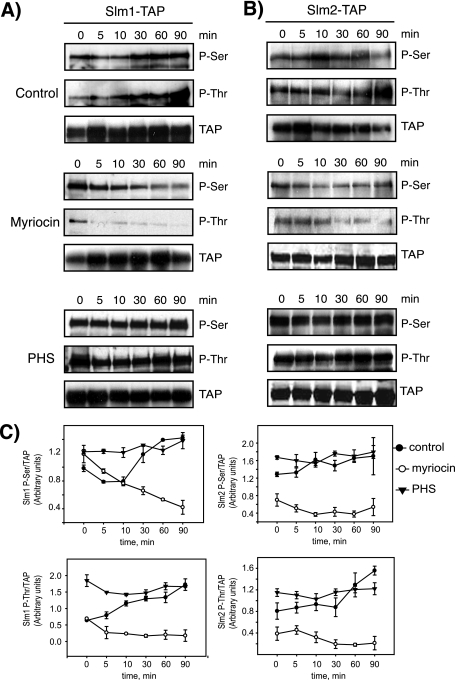
FIG. 4.
Slm1 and Slm2 phosphorylation in response to heat stress is dependent on sphingolipid synthesis. Western blot analysis of phosphorylated Slm1 (A) and Slm2 (B) proteins is shown. Cells expressing Slm1-TAP and Slm2-TAP were grown to mid-logarithmic phase in YPD medium at 26°C; aliquots of cells were then treated with vehicle alone (dimethyl sulfoxide), myriocin (2 μg/ml), or exogenous PHS (10 μM) for 30 min at RT and heat shock-shifted to 38°C for the indicated times. Cell extracts were prepared and subjected to TAP purification using IgG-Sepharose beads. Bound proteins were eluted with SDS-PAGE buffer, separated by SDS-PAGE, and immunoblotted with antibodies directed against phosphoserine (Q5) and phosphothreonine (Q7). Note that Slm2 Western blots were exposed to ECL reagents eight times longer than Slm1. (C) Quantitation of kinetics of Slm1 and Slm2 phosphorylation in response to heat stress in the presence or absence of drugs. Densitometric measurements of Western blots in panels A and B were made with the Image Gauge 4.0 program. Q5 and Q7 signals were normalized to the TAP signal for each time point. Results from two independent experiments are shown.