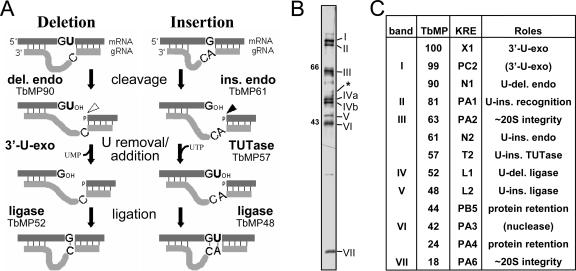
FIG. 1.
Mechanism of RNA editing and the purified editing complex. (A) Editing cycles, as described in the text, with the responsible enzymes indicated. U-deletional endonuclease (del. endo; cleavage shown with a hollow arrowhead) or U-insertional endonuclease (ins. endo; cleavage shown with a solid arrowhead) cleaves when the residue immediately upstream of the anchor duplex is a U or a purine, respectively, and is stimulated or inhibited by adenosine polyphosphates (8), such as AMP-CP. G and A represent either purine, and C represents a pyrimidine. (B) Silver staining of editing complex purified by Q-Sepharose and DNA cellulose chromatography, showing a different representative preparation from 667 cells than the four presented earlier (three in reference 38 and one other in reference 57), with seven major staining proteins that were designated band I (TbMP99) through band VII (TbMP18). (Band V [TbMP48] appears lighter upon photography because it silver stains brown, not black; band IV [TbMP52] has two isoforms in these cells [39]; and band III [TbMP63] exhibits microheterogeneity [20].) The asterisk indicates the position of the TbMP57 TUTase, identified by immunoblotting a comparable gel (57); that study also revealed that TbMP57 and band III (TbMP63) are present in the same relative abundance in total cell extract, indicating that TbMP57 was not lost during this purification. Size markers are in kDa. (C) Various nomenclatures for editing proteins. The columns show the original “band” designations from Rusche et al. (38), the TbMP designations (T. brucei mitochondrial protein, followed by the molecular weight of its cytoplasmic precursor) from Panigrahi et al. (33), and a more recent “KRE” nomenclature (kinetoplastid RNA editing, followed by a letter for the kind of protein and a number indicating ascending size within the group) (49). The literature, in addition to using these nomenclatures, also refers to TbMP52 as REL1 or DREL, TbMP48 as REL2 or IREL, and TbMP57 as TUT2. The final column shows a summary of the demonstrated (and proposed) roles in editing, as referenced in the Introduction; the role of TbMP18 is shown in this study.