Abstract
Muscle synaptogenesis in Drosophila melanogaster requires endocytosis of Commissureless (Comm), a binding partner for the ubiquitin ligase dNedd4. We investigated whether dNedd4 and ubiquitination mediate this process. Here we show that Comm is expressed in intracellular vesicles in the muscle, whereas Comm bearing mutations in the two PY motifs (L/PPXY) responsible for dNedd4 binding [Comm(2PY→AY)], or bearing Lys→Arg mutations in all Lys residues that serve as ubiquitin acceptor sites [Comm(10K→R)], localize to the muscle surface, suggesting they cannot endocytose. Accordingly, aberrant muscle innervation is observed in the Comm(2PY→AY) and Comm(10K→R) mutants expressed early in muscle development. Similar muscle surface accumulation of Comm and innervation defects are observed when dNedd4 is knocked down by double-stranded RNA interference in the muscle, in dNedd4 heterozygote larvae, or in muscles overexpressing catalytically inactive dNedd4. Expression of the Comm mutants fused to a single ubiquitin that cannot be polyubiquitinated and mimics monoubiquitination [Comm(2PY→AY)-monoUb or Comm(10K→R)-monoUb] prevents the defects in both Comm endocytosis and synaptogenesis, suggesting that monoubiquitination is sufficient for Comm endocytosis in muscles. Expression of the Comm mutants later in muscle development, after synaptic innervation, has no effect. These results demonstrate that dNedd4 and ubiquitination are required for Commissureless endocytosis and proper neuromuscular synaptogenesis.
Intensive investigations in recent years have ascribed a role for ubiquitination not only in protein degradation by the proteasome (14) but also in sorting and endocytosis of transmembrane proteins, often resulting in their degradation by the lysosome/vacuole (15). The attachment of a single ubiquitin (monoubiquitination) onto one or several cytoplasmic lysines in these transmembrane proteins serves as a signal for endocytosis or sorting (22, 31, 37, 45).
Nedd4 (neuronal precursor cell expressed developmentally downregulated 4), including the Drosophila melanogaster orthologue dNedd4, belongs to a family of Hect E3 ubiquitin ligases and is comprised of a C2 domain, three (or four) WW domains, and a C-terminal ubiquitin ligase Hect domain (39). The C2 domain is primarily involved in trafficking and membrane targeting (35, 36, 52), while the WW domains mediate protein:protein interaction and substrate recognition by binding to a short sequence called the PY motif (L/PPXY) (5, 18-20). Nedd4 proteins are involved in endocytosis and sorting of numerous transmembrane proteins. For example, in yeast, the Saccharomyces cerevisiae orthologue Rsp5 is required for ubiquitination, endocytosis, and vesicular/trans-Golgi network (TGN) sorting of the Fur4 and GAP1 permeases and the Ste2 receptor (8, 9, 12). In mammals, Nedd4 (or Nedd4-2) binds via its WW domains to the PY motifs of the epithelial Na+ channel (ENaC), promoting channel endocytosis and degradation (1, 46). These PY motifs are mutated in Liddle syndrome, a hereditary hypertension caused by increased retention and activity of ENaC at the cell surface of kidney epithelia (29, 41). Nedd4 is also involved in internalization of the IGF-1 receptor (51), in viral budding (26, 57), and in the ubiquitination of ubiquitin-interacting motif-containing endocytic proteins (e.g., Hrs and Eps15) (21, 37).
In Drosophila, dNedd4 was recently demonstrated to bind Commissureless (Comm) and to regulate internalization or sorting of the Comm/Roundabout (Robo) complex, thus promoting axon crossing at the central nervous system (CNS) midline (32). Comm is a single-pass transmembrane protein (49) that includes, at its cytoplasmic C terminus, two PY motifs (PPCY and LPSY), a YPSL motif (conforming to an AP-2 binding site), and 10 Lys residues (putative ubiquitin acceptor sites). Our recent work has demonstrated that dNedd4 binds via its WW domains to the PY motifs of Comm and that Comm is ubiquitinated by dNedd4 (18, 32).
In addition to its role in regulating axon midline crossing, Comm was previously shown to regulate neuromuscular synaptogenesis in Drosophila (55). In Drosophila, each body wall hemisegment contains a well-defined set of 30 muscles, arranged along the ventral-dorsal axis, that are innervated by about 40 motoneurons in a stereotypic, timed manner (25, 38, 42). All muscles of Drosophila embryos express Comm during the period of motoneuron-muscle interactions. However, Comm must be internalized into the muscle prior to synaptogenesis; comm mutants, or those lacking its cytoplasmic C terminus, fail to initiate synaptogenesis (55). The mechanism(s) by which Comm is endocytosed to regulate synaptogenesis has not been elucidated and is the focus of our current work.
In this study, we show that binding of Comm to dNedd4 and Comm ubiquitination mediate endocytosis of Comm and are required for muscle synaptogenesis.
MATERIALS AND METHODS
Generation of UAS-comm(WT), UAS-comm(2PY→AY), UAS-comm(2PY→AY-monoUb), UAS-comm(10K→R), UAS-comm(10K→R-monoUb) (all hemagglutinin [HA] tagged), UAS-dNedd4RNAi, and UAS-dNedd4(C→A) constructs (as per reference 4), as well as fly crosses, are detailed in the supplemental material.
Biochemical assays for expression of UAS-comm lines (WT and mutants) and UAS-dNedd4RNAi knockdown.
The UAS-comm (wild type [WT]; 2PY→AY, 2PY→AY-monoubiquitin [monoUb], 10K→R, 10K→R-monoUb) × daughterless-GAL4 embryos were collected at room temperature (RT) or 25°C, and the UAS-dNedd4RNAi × daughterless-GAL4 or UAS-dNedd4C→A embryos were collected at 29°C. The embryos were dechorionated with 50% bleach for 5 min and lysed with lysis buffer plus protease inhibitors (see Table S2 in the supplemental material). Wandering third-instar larvae from the UAS-dNedd4RNAi × daughterless-GAL4 crosses were also lysed as described above. Fillet preps of Oregon R third-instar larvae were prepared according to standard procedures (34) and lysed to examine endogenous expression of dNedd4 in the muscles by Western blotting (see Table S2 in the supplemental material). Expression of Comm-HA, dNedd4, and actin in these lysates were detected as detailed in Table S2 in the supplemental material.
Embryo and larva dissections and immunostaining.
For dNedd4 immunostaining, Oregon R wild-type embryos of different stages were collected at room temperature (RT), dechorionated, and fixed as described previously (34). Staining conditions are summarized in Table S2 in the supplemental material. For examination of embryonic synaptogenesis, stage 17 embryos were dissected as described previously (47), fixed in 4% paraformaldehyde (PFA) (20 min), washed with PBT (0.1% Tween 20 in phosphate-buffered saline), blocked, and stained with fluorescein isothiocyanate-conjugated anti-horseradish peroxidase (HRP) antibody (see Table S2 in the supplemental material). Muscle staining for HA or the extracellular domain of Comm (Comm-ECD) (10) was carried out as described in Table S2 in the supplemental material.
For larval immunostaining, wandering third-instar larvae were dissected using a standard fillet method (2) and fixed as described above for embryos. Cy3 anti-HRP or fluorescein isothiocyanate-anti-HRP staining was performed as described in Table S2 in the supplemental material. Abdominal segments 2 to 6 were scored for the presence/absence of mutant muscle synaptic phenotypes. Anti-HA, Hrs (antibodies kindly provided by H. Bellen (30), or dNedd4 staining of larval muscles was carried out as described previously (see Table S2 in the supplemental material). Epifluorescence microscopy and imaging of the hemisegments was performed using a Leica IRE2 microscope and an LSM 510 confocal microscope.
S2 cells immunostaining and RNA interference (RNAi). (i) Comm endocytosis.
Live S2 cells expressing Comm-green fluorescent protein (GFP) were incubated with affinity-purified and embryo (chorionated)-precleared anti-Comm-ECD antibody for 60 min (RT). Cells were then washed, fixed in 2% PFA (10 min), permeabilized with 0.1% Saponin, and stained with secondary antibody (see Table S2 in the supplemental material). Immunostaining of fixed and permeabilized S2 cells with lysobisphosphatidic acid (LBPA) (27), dRab5 (56), or transfected myc-tagged liquid facets were performed as listed in Table S2 in the supplemental material. Endogenous Hrs was stained with anti-Hrs antibodies (see Table S2 in the supplemental material). Immunostained cells were visualized with an LSM 510 confocal microscope.
(ii) RNAi for dNedd4.
A 638-bp fragment (nucleotides 435 to 1072; FlyBase data bank accession no. CG7555) was subcloned as an inverted repeat (IR) into pBluescript. The in vitro transcription dNedd4-IR was driven from either the T3 or T7 promoter to yield the sense and anti-sense RNA, respectively, using mMESSAGE mMACHINE (Ambion). The mRNA was then annealed to form the dNedd4-double-stranded RNA hairpin (dNedd4-RNAi), and S2 cells, transfected (or not) with Comm-GFP, were treated with dNedd4-RNAi as described previously (7). Knockdown of dNedd4 in lysed cells was confirmed by immunoblotting with anti-dNedd4 antibodies and levels of Comm-GFP with anti-GFP antibodies (see Table S2 in the supplemental material). Samples of cells were also scored for cell surface localization of Comm.
Ubiquitination assays.
S2 cells were transiently transfected with myc-tagged Comm [WT, 2PY→AY, 10K→R, or dNedd4(C→A)] in the pRmHA3 vector (32), pretreated with proteasome and lysosome inhibitors (50 μM N-acetyl-l-leucinal-l-leucinal-l-nor leucinal [LLnL] or 20 μM MG101 and 0.4 mM chloroquine), and lysed in lysis buffer containing protease inhibitors (see Table S2 in the supplemental material) plus 50 μM LLnL and 0.4 mM chloroquine. The Comm (WT or mutants) protein was immunoprecipitated (IP) with anti-myc antibodies (1.5 μg/ml) and blotted with antiubiquitin antibodies (see Table S2 in the supplemental material).
RESULTS
comm mutants unable to bind dNedd4 or to become ubiquitinated show defects in endocytosis and muscle synaptogenesis along the SNb nerve branch. (i) Defective ubiquitination of the PY motif and Lys mutants of Comm.
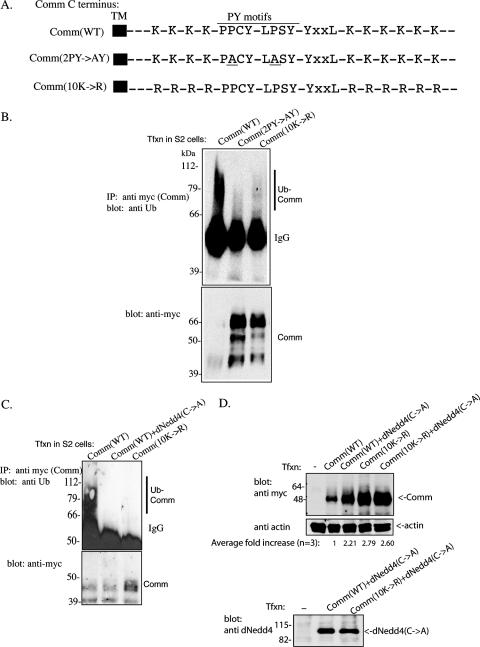
Our previous work has demonstrated that mutations in the two PY motifs of Comm (2PY→AY) that abolish binding to dNedd4 or in its 10 cytoplasmic Lys residues (10K→R) that potentially serve as ubiquitin acceptor sites (Fig. 1A) lead to impaired endocytosis when expressed in Drosophila S2 cells (32). To test whether these mutants are indeed defective in ubiquitination, myc-tagged Comm(WT), Comm(2PY→AY), or Comm(10K→R) was expressed in Drosophila S2 cells. The Comm protein (WT or mutant) was then IP with anti-myc antibodies, and the IP was immunoblotted with anti-ubiquitin antibodies to detect ubiquitination of Comm. As seen in Fig. 1B, unlike wild-type Comm, Comm that cannot bind dNedd4 or has all its cytoplasmic lysines mutated to arginines shows impaired ubiquitination, which contributes to stabilization of the Comm mutant protein (Fig. 1B, bottom, and D).
FIG. 1.
Loss of ubiquitination of the Comm(2PY→AY) and Comm(10K→R) mutants. (A) Schematic representation of the C terminus of Comm, showing its 10 Lys residues, two PY motifs, and the YXXL motif. Also shown are the Comm(2PY→AY) and Comm(10K→R) mutants. (Only relevant residues are shown, with spacing between them not to scale.) TM, transmembrane. (B) Drosophila S2 cells were transfected (Tfxn) with myc-tagged wild-type Comm [Comm(WT)], Comm bearing mutations in the two PY motifs to abolish binding to dNedd4 [Comm(2PY→AY], or Comm mutated in all 10 of its cytoplasmic Lys residues [Comm(10K→R] that serve as putative ubiquitin acceptor sites. Comm (WT or mutants) was then immunoprecipitated (IP) from cells, and the IPs were immunoblotted with antiubiquitin (Ub) antibodies to detect ubiquitinated Comm (upper panel). Comm expression levels in the lysates were verified with anti-myc antibodies (lower panel). Note that a substantial fraction of Comm(WT), which is ubiquitinated, disappears (likely degraded), whereas the Comm mutants are stabilized. (C) The same as panel B, only also showing loss of Comm(WT) ubiquitination by endogenous dNedd4 in S2 cells by overexpression of catalytically inactive dNedd4(C→A). Comm protein here (and in other figures) appears as multiple bands, which is the pattern normally observed with Comm, and may represent posttranslational modifications (see reference 23). (D) Increased stability of ubiquitination-impaired Comm [(Comm(10K→R)] or WT Comm coexpressed with catalytically inactive dNedd4(C→A). S2 cells transfected (or not) with myc-tagged Comm(WT) or Comm(10K→R) alone or with Flag-dNedd4(C→A) were lysed, and lysates were analyzed by Western blotting for the amounts of Comm present. Control lower panels depict actin and dNedd4(C→A) expression. The average increase in Comm stability from three independent experiments is indicated as well. IgG, immunoglobulin G.
(ii) Defects in endocytosis of mutant Comm in the muscle.
Earlier work has demonstrated that removal of Comm from the surface of muscles in Drosophila is required for initiation of synaptogenesis. comm mutants lacking the intracellular C terminus do not properly internalize and show defects in synaptogenesis (55). The intracellular C terminus of Comm contains two PY motifs (PPCY and LPSY) that serve as binding sites for the WW domains of dNedd4 as well as 10 Lys residues that serve as ubiquitin acceptor sites (Fig. 1A) (18, 32). To test whether binding of dNedd4 to Comm and Comm ubiquitination could be responsible for its internalization, we generated UAS lines that overexpress triple HA-tagged Comm (tagged at the C terminus) WT(+), 2PY→AY, or 10K→R mutants (see Table S1 in the supplemental material). Figure S1 in the supplemental material shows that the WT and mutant comm lines express the protein when driven by daughterless-GAL4, which is ubiquitously expressed in Drosophila. These UAS lines were then used for our further studies.
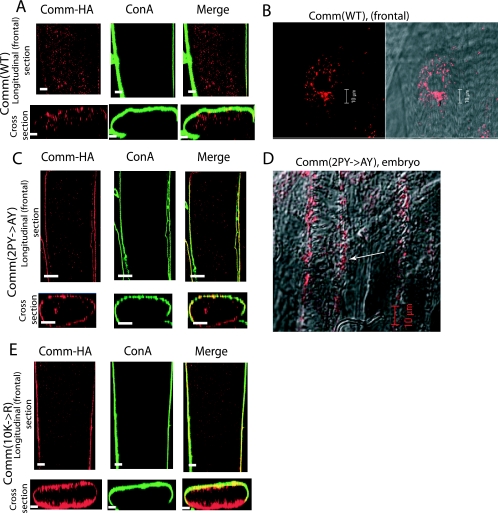
We next examined the distribution of WT and mutant Comm in the muscle by crossing the UAS-comm lines with the early muscle driver 24B-GAL4, which drives expression from embryonic stage 11 (before the onset of synaptogenesis) until the end of the larval third instar (4, 11), and staining for Comm with anti-HA antibodies. As seen in Fig. 2A and B, the expression of Comm(WT) in muscles from third-instar larvae appears in intracellular vesicles. This pattern of distribution is observed in all muscles of all the hemisegments and is in accord with earlier work with endogenous Comm where Comm was shown to enter the endosomes from the muscle surface by 18 h (stage 17) of embryonic development (55).
FIG. 2.
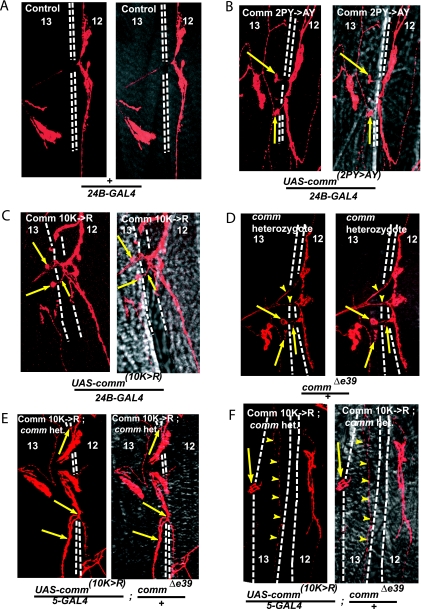
Cellular localization of HA-tagged WT and mutant Comm in muscles. Longitudinal and cross-section confocal images of HA-tagged Comm (WT and mutants) expressed in muscle 12 or 13 of third-instar larvae that were costained with the plasma membrane marker concanavalin A (ConA). (A and B) Expression of Comm(WT) in UAS-comm+(WT)/24B-GAL4 larvae (A), with higher magnification shown in panel B, revealing vesicular expression of Comm(WT). (C and D) Expression of Comm(2PY→AY) in UAS-comm2PY→AY/24B-GAL4 larvae (C) or embryos (D). (E) Expression of Comm(10K→R) in UAS-comm10K→R/24B-GAL4 larvae. Note the accumulation of mutant Comm [Comm(2PY→AY) and Comm(10K→R)] at the plasma membrane of the muscle, while Comm(WT) accumulates in intracellular vesicles. Scale bars, 10 μm.
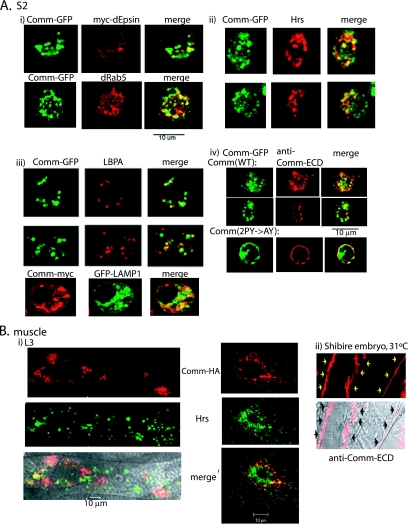
To identify these Comm-containing intracellular vesicles, we performed coimmunostaining analyses in both S2 cells and larval muscles from UAS-comm+/24B-GAL4 flies. As shown in Fig. 3, partial colocalization of Comm with dEpsin (liquid facets) (a marker for the plasma membrane [PM] and coated pits) (6, 53), dRab5 (early/sorting endosome marker), Hrs (a marker for early and maturing endosomes), or lysobisphosphatidic acid (LBPA) and LAMP1 (markers for late endosomes/lysosome) suggests that the Comm-containing vesicles represent a collection of endocytic vesicles at different stages of maturation.
FIG. 3.
WT Comm internalizes from the plasma membrane and colocalizes with endocytic markers. (A) Drosophila S2 cells were cotransfected with Comm-GFP and myc-dEpsin, immunostained with anti-myc antibodies, and analyzed by confocal microscopy for colocalization of Comm and dEpsin at the PM and in early endosomes. Colocalization of Comm-GFP with the early endosomal marker dRab5 is shown as well (panel i). Panel ii depicts colocalization of endogenous Hrs (marking early and maturing endosomes) and transfected Comm-GFP in S2 cells, and panel iii depicts colocalization of endogenous LBPA or transfected LAMP1 (marking late endosomes/lysosomes) with transfected Comm in these cells. Panel iv demonstrates immunostaining with antibodies directed to the extracellular domain (ECD) of Comm of live (nonpermeabilized) S2 cells transfected with Comm-GFP. Note the accumulation of WT Comm in vesicles following their internalization from the PM (upper panel) and the accumulation of the Comm(2PY→AY) mutant at the PM (lower panel). (B) Panel i shows muscles dissected from third-instar larvae of UAS-comm+/24B-GAL4 flies and coimmunostained with anti-HA antibodies (to detect the HA-tagged Comm) and anti-Hrs antibodies and analyzed by confocal microscopy. The left panel depicts a muscle section, and the right panel represents a higher magnification of a muscle segment. The bottom left panel shows a confocal image overlaid over a differential interference contrast image. Panel ii shows endogenous Comm, stained with anti-Comm-ECD, accumulating in muscle surface of stage 17 embryos of shibire mutants grown at the nonpermissive temperature (31°C) to block internalization of PM proteins. Arrows mark the surface of two muscles.
In contrast to Comm(WT), the expression of Comm(2PY→AY), Comm(10K→R) (UAS-comm2PY→AY/24B-GAL4, or UAS-comm10K→R/24B-GAL4) appears confined to the muscle surface, with little or no observed intracellular vesicular staining (Fig. 2C and E). This was seen in muscles of both third-instar larvae (Fig. 2C) and stage 17 embryos (Fig. 2D). These results suggest that endocytosis of Comm in the muscle requires that dNedd4 binds and ubiquitinates Comm.
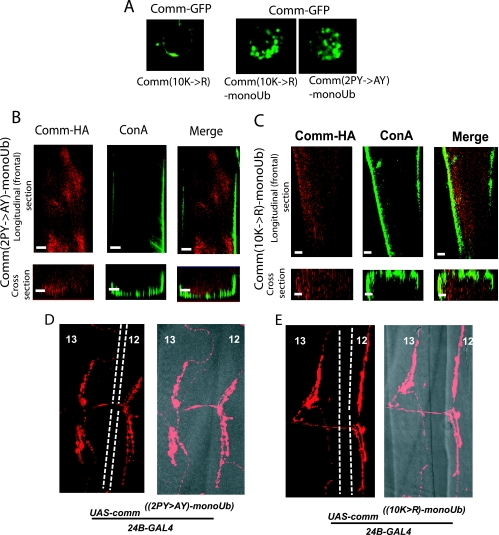
Recent work by Keleman et al. (23), using transfection in mammalian Cos-7 cells, has suggested that WT Comm is sorted to endosomes from the Golgi without ever reaching the PM. To investigate the route by which WT Comm localizes to endocytic vesicles in both transfected Drosophila S2 cells and in embryo muscles (which express endogenous Comm), we used an antibody directed to the extracellular domain of Comm (Comm-ECD) (10). As seen in Fig. 3A, panel iv, surface labeling of live S2 cells expressing Comm-GFP with anti-Comm-ECD antibodies (followed by antibody removal, permeabilization, and staining with secondary antibodies) demonstrates that labeled WT Comm accumulates in endocytic vesicles, suggesting that Comm was present at the PM prior to its internalization. This vesicular accumulation of surface-labeled Comm was seen in >95% of cells analyzed (n = ∼100). No significant staining was detected in untransfected cells (data not shown), suggesting that fluid-phase endocytosis of the anti-Comm-ECD antibodies is negligible. As expected, Comm that is unable to bind dNedd4 [Comm(2PY→AY)] accumulates at the PM (Fig. 3A, panel iv). Importantly, analysis of the distribution of endogenous Comm expressed in the muscles of shibire (Drosophila dynamin) mutant embryos at the nonpermissive temperature (31°C), in which internalization of PM proteins is blocked, reveals the accumulation of Comm exclusively at the muscle surface (Fig. 3B, panel ii) and was observed in 100% of muscle analyzed (n = ∼60). Collectively, these results suggest that a substantial fraction of WT Comm normally traffics to the PM prior to its internalization into vesicles in both S2 and Drosophila muscle cells.
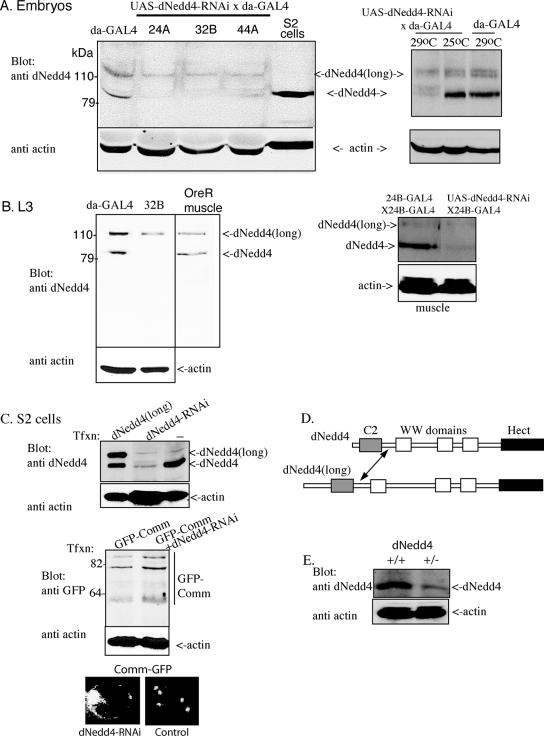
To verify that dNedd4 is expressed endogenously in the muscles, we immunostained muscles with anti-dNedd4 antibodies (characterized in Fig. 6C). Figure S2 in the supplemental material shows that dNedd4 is uniformly expressed in all muscles in an apparent cytoplasmic distribution, resembling the pattern of other cytoplasmic muscle proteins (28). This distribution could accommodate interactions of dNedd4 with the C terminus of Comm (where the two PY motifs are located), which is cytoplasmic. The distribution of dNedd4 in the embryo is ubiquitous and is apparent from early to later stages of development (see Fig. S2B to F in the supplemental material), including the time period when Comm is expressed in the embryo (55). Similar ubiquitous distribution of dNedd4 mRNA in embryos was observed by RNA in situ hybridization (data not shown).
FIG. 6.
Knockdown of dNedd4 in S2 cells and in embryos and larvae overexpressing UAS-dNedd4RNAi. (A) Expression of endogenous dNedd4 and its splice variant dNedd4(long) in lysates of daughterless-GAL4 (da-GAL4) embryos and knockdown of these variants in three separate lines (24A, 32B, and 44A; integrated on chromosomes X, III, and II, respectively) at 29°C (left panel). Lysate from S2 cells, which express endogenous dNedd4, is shown as well. The right panel demonstrates knockdown of dNedd4 by RNAi at 29°C but not at 25°C. (B) dNedd4 knockdown is maintained during the third-instar (L3) larval stage as well. The middle panel depicts endogenous expression of dNedd4 and dNedd4(long) in wild-type Oregon R (OreR) larval muscles. The right panel depicts knockdown of dNedd4 in larval muscle following expression of UAS-dNedd4RNAi driven by 24B-GAL4 (at 29°C). Densitometry analysis of the bands in the figure reveals almost complete knockdown of dNedd4 and ∼40% knockdown of dNedd4(long) relative to the actin controls. This experiment was repeated three times with similar results. (C) Knockdown of endogenous dNedd4 in S2 cells. Transfected dNedd4(long) and endogenous dNedd4 are shown in the left lane as controls. The middle panel shows that knockdown of dNedd4 with RNAi leads to increased amounts of Comm. The bottom panel shows accumulation of Comm at the plasma membrane of S2 cells following knockdown of endogenous dNedd4 with RNAi. There was an ∼5-fold increase (n = 360) in the frequency of cells that accumulate Comm following treatment with dNedd4-RNAi relative to controls. (D) Schematic representation of dNedd4 and dNedd4(long), with the arrow pointing to the region shared by these two splice isoforms that is recognized by the anti-dNedd4 antibodies. (E) Flies heterozygous for dNedd4 (+/−) express reduced amounts of endogenous dNedd4 relative to control (+/+) flies.
(iii) Muscle innervation defects in Drosophila overexpressing Comm(2PY→AY) and Comm(10K→R) mutants.
To study muscle innervation, we overexpressed the Comm(2PY→AY) and Comm(10K→R) mutants in muscles early in development, adopting the same approach previously utilized to test the effect of C-terminally truncated Comm on synaptogenesis (55). Innervation of the body wall muscles in the larva is highly stereotypical, and in particular the innervation of muscles 12 and 13 by the SNb branch of the segmental nerve has been well characterized (16, 17, 43, 50) (Fig. 4A; also see Fig. S3 in the supplemental material). The innervation pattern of +/24B-GAL4 (Fig. 4A) resembles the stereotypical innervation patterns of muscles 12 and 13 (48, 50). Some aberrant innervation was sometimes seen in WT Comm (20% frequency; Table 1). This likely reflects the exquisite sensitivity of muscle innervation to Comm dosage (see below).
4.
Aberrant muscle innervation along the SNb branch in larvae overexpressing Comm(2PY→AY) and Comm(10K→R). Confocal analysis of Cy3 anti-HRP staining of motoneurons innervating muscles 12 and 13 of third-instar larvae grown at room temperature from the following crosses: (A) 24B-GAL4 (+/24B-GAL4); (B) UAS-comm2PY→AY/24B-GAL4; (C) UAS-comm10K→R/24B-GAL4; and (D) comm/+. Note the abnormal branching (arrowheads). (E and F) UAS-comm10K→R expressed in comm heterozygote background (UAS-comm10K→R/5-GAL4 commΔe39/±). (F) Note that only small type II boutons innervate muscle 13 (arrowheads), with a “stalled” axon primarily innervating the neighboring muscle (arrow), leaving the muscle largely devoid of innervation. Where indicated, the second chromosome embryonic muscle driver 5-GAL4 was used for the crosses (55), which allows expression in a comm-reduced background (comm is located on the third chromosome). (B to E) Arrows indicate aberrant innervation of muscle 13 in the comm mutants. Right-hand images in each panel depict the fluorescent (confocal) image overlaid onto a differential interference contrast image of the muscle. Dashed white lines represent the border separating muscles 12 and 13. Het denotes heterozygote.
TABLE 1.
Frequency of ectopic innervation or pathfinding errors in the SNb nerve branch onto muscles 12 and 13 in larvae overexpressing Comm or its mutantsa
| Genotype | n | Frequency of abnormal innervation (%) |
|---|---|---|
| UAS-comm [2PY→AY]/24B-GAL4 | 83 | 42.2b |
| UAS-comm [10K→R]/24B-GAL4 | 88 | 35.2b |
| UAS-comm+/24B-GAL4 (control 1) | 65 | 20.0c |
| +/24B-GAL4 (control 2) | 76 | 3.9 |
| UAS-comm [(2PY→AY)-monoUb]/24B-GAL4 | 97 | 19.6 |
| UAS-comm [(10K→R)-monoUb]/24B-GAL4 | 86 | 23.3 |
| commLOF/+ | 108 | 26.9 |
| UAS-comm10K→R/5-GAL4; commΔe39/+ | 72 | 47.2 |
The following P values were determined by Fisher's exact test (two-tailed): 2PY→AY versus WT Comm (control 1), P < = 0.0048; 10K→R versus WT Comm (control 1), P < = 0.0475; 2PY→AY versus +/24B-GAL4 (control 2), P < = 0.0001; 10K→R versus +/24B-GAL4 (control 2), P < = 0.0001; WT Comm (control 1) versus +/24B-GAL4 (control 2), P < = 0.0032; 10K→R-monoUb versus 24B-GAL4 (control 2), P < = 0.0005; 2PY→AY-monoUb versus 24B-GAL4 (control 2), P < = 0.0041; 10K→R-monoUb versus Comm WT (control 1), P < = 0.84; 2PY→AY-monoUb versus Comm WT (control 1), P < = 0.6938; 10K→R in comm heterozygote background versus comm heterozygote, P < = 0.0066; 10K→R in comm heterozygote background versus 10K→R in WT background, P < = 0.1464.
Significance was at the P = 0.05 level with respect to the +/24B-GAL4 and UAS-comm+/24B-GAL4 controls.
Significance was at the P = 0.05 level with respect to the +/24B-GAL4 control.
In contrast, highly significant increases in aberrant innervation at muscles 12 and 13 relative to +/24B-GAL4 and UAS-comm+/24B-GAL4 controls were observed when UAS-comm(2PY→AY) and UAS-comm(10K→R), driven by 24B-GAL4, were overexpressed in the muscles (impaired innervation in 42% and 35% of all muscles, respectively [Table 1]). The overexpression of these two mutants caused similar synaptic phenotypes, whereby ectopic boutons/collaterals originating from muscle 12 crossed the cleft separating muscles 12 and 13, and incorrectly innervated muscle 13 (Fig. 4B and C; also see Fig. S3 in the supplemental material). These neurons normally do not cross this cleft (Fig. 4A). This suggests that the PY motifs and the ubiquitin acceptor sites of Comm are required for normal synaptogenesis.
The timing of Comm (WT or mutant) expression in the muscle is critical, as the synaptogenesis defects seen (Fig. 4) are only observed in UAS-comm (2PY→AY or 10K→R) driven by 24B-GAL4 (expressed during embryogenesis) but not when driven by the Mhc-GAL4 driver, which is expressed later in development (first- to third-instar larvae) (data not shown).
(iv) Monoubiquitination of Comm is sufficient to promote Comm endocytosis and to restore normal synaptogenesis in comm mutants.
Mono- or multimonoubiquitination has been shown to mediate endocytosis/sorting of several transmembrane proteins. To test whether monoubiquitination of Comm is sufficient to induce its internalization from the muscle surface and to prevent the synaptogenesis defects seen with the Comm(2PY→AY) and Comm(10K→R) mutants, we fused these mutant constructs in frame with a single mutant ubiquitin that itself cannot become polyubiquitinated (K11,29,48,63R, G75,76A-ubiquitin, hereafter called monoUb). As seen in Fig. 5A, unlike the plasma membrane distribution of the Comm(2PY→AY) (Fig. 3A, panel iv) or Comm(10K→R) (Fig. 5A) mutant, the Comm(2PY→AY-monoUb) or Comm(10K→R-monoUb) mutant regained its ability to internalize in S2 cells (Fig. 5A), much like WT Comm (Fig. 3A, panel iv). Accordingly, UAS fly lines generated from these monoUb constructs (see Table S1 and Fig. S1 in the supplemental material) revealed a restoration of Comm endocytosis from the muscle surface when crossed to 24B-GAL4 (Fig. 5B and C), much like WT-comm but unlike the comm(2PY→AY) or comm(10K→R) mutants, which were retained at the cell surface. Importantly, the synaptogenesis defects seen with the comm(2PY→AY) or comm(10K→R) mutant larvae were no longer observed in the comm(2PY→AY-monoUb) and comm(10K→R-monoUb) larvae crossed to 24B-GAL4 lines (Fig. 5D and E; Table 1). These results suggest that monoubiquitination of Comm is sufficient to promote Comm endocytosis and to prevent the synaptogenesis defects seen with the comm(2PY→AY) or comm(10K→R) mutants.
FIG. 5.
Monoubiquitination of mutant comm restores Comm endocytosis and normal synaptogenesis. (A) Vesicular distribution of GFP-tagged Comm(2PY→AY-monoUb) and Comm(10K→R-monoUb) (right panels). The left panel depicts an example of plasma membrane localization of Comm(10K→R); (B) expression of Comm(2PY→AY-monoUb) in UAS-comm2PY→AY-monoUb/24B-GAL4 larvae; (C) expression of Comm(10K→R-monoUb) in UAS-comm10K→R-monoUb/24B-GAL4 larvae. Scale bars, 10 μm. (D and E) Lack of innervation defects in (D) UAS-comm2PY→AY-monoUb/24B-GAL4 and (E) UAS-comm10K→R-monoUb/24B-GAL4. Note the normal innervation pattern of the comm mutants fused to monoUb. The right-hand images in each panel depict the fluorescent (confocal) image overlaid onto a differential interference contrast image of the muscle. Dashed white lines represent the border separating muscles 12 and 13.
(v) Analysis of synaptogenesis by the comm mutants in comm-null or comm/+ background.
In the above studies, the comm mutants were expressed in muscles in the background of normal amounts of endogenous Comm. We thus tested whether the synaptogenesis defects observed in the comm mutants are also seen in the background of reduced levels of endogenous Comm. The comm deficiency is lethal during embryogenesis. However, in comm/+ larvae (expressing half the Comm levels), significant synaptogenesis defects were observed (∼27% defects relative to 8% in the controls) (Fig. 4D; Table 1), in agreement with earlier work (55). Expressing UAS-comm(2PY→AY) or UAS-comm(10K→R) in the muscle of the comm-null mutants could not rescue the lethality. However, expression of UAS-comm(10K→R) in a comm/+ (heterozygote) background permitted larval development but revealed severe synaptogenesis defects (∼50% relative to 35% in a WT genetic background) (Fig. 4E and F; Table 1). Interestingly, while muscular expression of UAS-comm(2PY→AY) in the comm/+ background could not rescue the lethality, such rescue was observed upon expression of UAS-comm(2PY→AY)-monoUb. These results support our findings described above (Fig. 4B and C), implicating the Comm PY motifs and Comm ubiquitination in muscle synaptogenesis.
Reduction of dNedd4 protein and function in the muscles results in aberrant synaptic phenotypes along the SNb branch. (i) Knockdown of dNedd4 by RNAi leads to synaptogenesis defects.
The observation that the Comm(2PY→AY) mutant, which cannot bind dNedd4, shows muscle innervation defects when expressed early in muscle development, coupled with the demonstration that endogenous dNedd4 ubiquitinates Comm in S2 cells (Fig. 1B and C), suggests that dNedd4 may mediate the ubiquitination and endocytosis of Comm in muscles. To reduce muscular dNedd4 expression, we used RNA interference (RNAi) to knock down expression of endogenous dNedd4 in the muscle. We first verified that our dNedd4-RNAi approach is effective by demonstrating knockdown of endogenous dNedd4 in S2 cells (Fig. 6C, top) and increased stabilization of Comm (Fig. 6C, middle) and accumulation of Comm at the plasma membrane of these cells (Fig. 6C, bottom). We then generated UAS-dNedd4RNAi flies. To verify that endogenous dNedd4 is indeed knocked down in flies ubiquitously overexpressing this construct, we generated UAS-dNedd4RNAi/daughterless-GAL4 flies (see Table S1 in the supplemental material) and tested dNedd4 expression in embryo and larva lysates. As seen in Fig. 6A and B, dNedd4 in embryos and larvae is observed as two protein bands (splice isoforms), dNedd4 and dNedd4(long) (Fig. 6D), and the same two bands are also observed in the muscles of WT OreR larvae (Fig. 6B, right panel). Both splice isoforms are recognized by our anti-dNedd4 antibodies, directed at a shared sequence downstream of the C2 domain (Fig. 6D, arrow). Figure 6A shows that overexpression of UAS-dNedd4RNAi by daughterless-GAL4 (in three different lines, each inserted on a different chromosome) at 29°C (but not at 25°C; see the right panel) knocked down most of the endogenous dNedd4 and ∼40% of dNedd4(long) protein both in embryos (Fig. 6A, left panel) and in third-instar larvae (Fig. 6B). DNedd4 knockdown was also seen in larval muscles following overexpression of UAS-dNedd4RNAi by 24B-GAL4 to knock down dNedd4 specifically in the muscle (Fig. 6B, right panel). The less efficient knockdown of dNedd4(long) by the RNAi approach, although targeting a sequence shared by both splice variants, may be due to the longer distance of the targeted sequence from the N-terminal start of the gene.
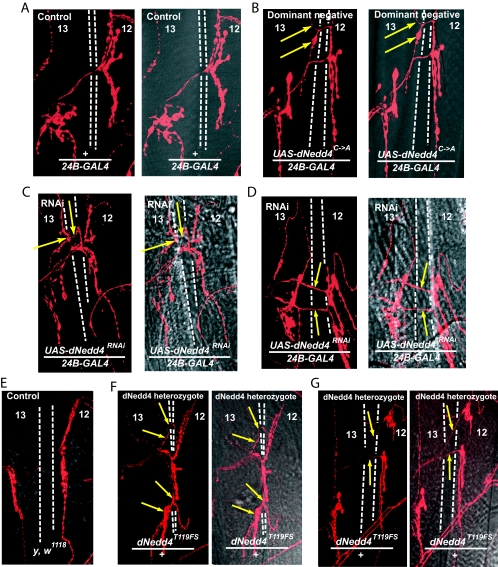
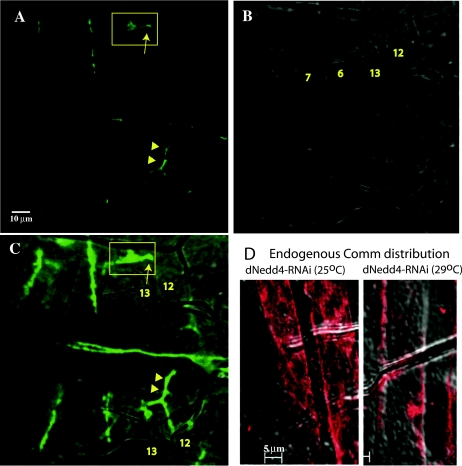
We next tested the effect of knocking down dNedd4 on muscle synaptogenesis by expressing UAS-dNedd4RNAi in early muscle development using the 24B-GAL4 driver. Our results, depicted in Fig. 7, Table 2, and Fig. S4 in the supplemental material, demonstrate that RNAi-mediated knockdown of muscular dNedd4 expression yields synaptic mutant phenotypes similar to those observed with the Comm(2PY→AY) or the Comm(10K→R) mutant. The most obvious defect is the abnormal innervation patterns seen at muscles 12 and 13 (ectopic boutons/collaterals innervating muscle 13; Fig. 7C and D), observed at a frequency of ∼21% (compared to 6% for the UAS-dNedd4RNAi and 7% for the +/24B-GAL4 controls) (Table 2). Another occasionally observed defect is abnormal branching of the nerve-innervating muscle 12 (Fig. 7D, arrows). The abnormal innervation pattern of muscles 12 and 13 seen in larval muscle likely originated earlier during embryonic development, as it can already be detected in muscles of stage 17 embryos (Fig. 8A to C). Interestingly, as apparent from Fig. 8D, these innervation defects correlated with increased accumulation of endogenous Comm at the surface of muscles of embryos in which dNedd4 was knocked down by RNAi at 29°C (where dNedd4 knockdown is observed) but not at 25°C (where knockdown is not seen [Fig. 6A]). In support of the dNedd4-RNAi findings, reduction in half of the endogenous dNedd4 in dNedd4/+ heterozygotes, kindly provided by S. Hayashi (40) (Fig. 6E), led to defects in muscle synaptogenesis similar to those seen with knockdown of dNedd4 with RNAi (Fig. 7E; Table 3), although at a lower frequency, likely due to the smaller reduction of dNedd4 levels in the dNedd4/+ embryos relative to dNedd4-RNAi (Fig. 6). (The dNedd4 null is lethal and could not be analyzed.) Note how the dNedd4/+ phenotypes in Fig. 7F (ectopic boutons on muscle 13) and G (abnormal branching) recapitulate the dNedd4-RNAi phenotypes observed in Fig. 7C and D, respectively.
FIG. 7.
Reduction of dNedd4 levels in the muscle by RNAi or heterozygosity, or overexpression of a catalytically inactive dNedd4 [dNedd4(C→A] during embryonic development, leads to muscle innervation defects along the SNb branch. Confocal analysis of Cy3 anti-HRP staining of motoneurons innervating muscles 12 and 13 of third-instar larvae is shown. (A) Normal innervation in control WT larvae (+/24B-GAL4); (B) innervation defects in UAS-dNedd4C→A/24B-GAL4 larvae expressing a dominant-negative dNedd4 in the muscle. (C and D) UAS-dNedd4RNAi/24B-GAL4 larvae in which dNedd4 is knocked down in the muscle. (E) y, w1118 control for dNedd4/+. (F and G) Innervation defects observed in dNedd4/+ heterozygotes (using dNedd4T119FS, a loss-of-function allele [40]). Arrows depict the path of the misguided neuron in panels B, C, and F. Arrows indicate abnormal branching in panels D and G. Crosses were grown at 29°C.
TABLE 2.
Frequency of ectopic innervation or pathfinding errors of the SNb nerve branch onto muscles 12 and 13 in larvae knocked down for dNedd4 with RNAi
| Genotype | n | Frequency of abnormal innervationa (%) |
|---|---|---|
| UAS-dNedd4RNAi/24B-GAL4 | 160 | 21.3b |
| UAS-dNedd4RNAi (control 1) | 100 | 6.0 |
| +/24B-GAL4 (control 2) | 181 | 7.2 |
P values determined by Fisher's exact test (two-tailed) were the following: versus control 1 (UAS-dNedd4RNAi), P < = 0.0007; versus control 2 (+/24B-GAL4), P < = 0.0002; control 1 versus control 2, P < = 0.8076.
Significance was at the P = 0.05 level with respect to both controls.
FIG. 8.
Detection of muscle innervation defects along the SNb branch in embryos following knockdown of dNedd4 by RNAi. (A to C) Innervation defects on muscles 12 and 13 in UAS-dNedd4RNAi/24B-GAL4 stage 17 embryos grown at 29°C. Arrow in the boxed areas depict stalling of innervation of muscle 12 compared to normal innervation patterns shown below (arrowheads). (A) Confocal image; (B) DIC image; (C) overlay of the two images. (D) Confocal images (overlaid on a differential interference contrast image) of muscles from stage 17 embryo grown at 25°C (left) or 29°C (right) and immunostained with anti-Comm-ECD. Note the intracellular (intramuscle) distribution of endogenous Comm at 25°C (where there is no knockdown of dNedd4) and surface accumulation of Comm at 29°C (where dNedd4 is knocked down). Scale bars, 10 μm in panel A and 5 μm in panel D.
TABLE 3.
Frequency of ectopic innervation or pathfinding errors of the SNb nerve branch onto muscles 12 and 13 in dNedd4/+ heterozygous larvae
| Genotype | n | Frequency of abnormal innervation (%) |
|---|---|---|
| y w; +; dNedd4T119FS/+ | 210 | 12.9 |
| y w1118 (control) | 174 | 7.4 |
Taken together, these results demonstrate that reduced expression of dNedd4 by RNAi (or heterozygosity) in muscles innervated by the SNb nerve branch causes synaptogenesis defects similar to those seen in the Comm 2PY→AY or 10K→R mutants and is correlated with increased frequency of Comm accumulation at the muscle surface.
(ii) Overexpression of catalytically inactive dNedd4(C→A) in the muscle results in synaptic defects similar to those seen with dNedd4-RNAi.
Figure 1C shows that a catalytically inactive dNedd4(C→A), bearing a Cys-to-Ala mutation in the conserved Cys of the Hect domain, is unable to ubiquitinate Comm, and moreover, it diminishes ubiquitination by endogenous dNedd4 in S2 cells, stabilizing Comm (Fig. 1D) and hence acting in a dominant-negative fashion. Therefore, overexpression of dNedd4(C→A) in Drosophila muscles is expected to interfere with Comm ubiquitination by endogenous dNedd4. This could be functionally similar to knockdown of endogenous dNedd4 by RNAi. As seen in Fig. 7B and Fig. S4 in the supplemental material, analysis of animals that overexpress dNedd4(C→A) in the muscles reveals synaptic defects comparable to those seen with dNedd4-RNAi knockdown, demonstrating aberrant innervation patterns at muscles 12 and 13. These abnormalities are quantified in Table 4, which demonstrates ∼18% abnormal innervation of muscles 12 and 13 in flies overexpressing mutant dNedd4(C→A) (UAS-dNedd4C→A/24B-GAL4) relative to ∼3% and 5% defects in the UAS-dNedd4C→A and +/24B-GAL4 controls.
TABLE 4.
Frequency of ectopic innervation or pathfinding errors of the SNb nerve branch onto muscles 12 and 13 in larvae overexpressing dNedd4C→A
| Genotype | n | Frequency of abnormal innervationa (%) |
|---|---|---|
| UAS-dNedd4C→A/24B-GAL4 | 146 | 17.8b |
| UAS-dNedd4C→A (control 1) | 142 | 2.8 |
| +/24B-GAL4 (control 2) | 187 | 5.3 |
P values determined by Fisher's exact test (two-tailed) were the following: versus control 1 (UAS-dNedd4C→A), P < = 0.0001; versus control 2 (+/24B-GAL4), P < = 0.0003; control 1 versus control 2, P < = 0.2872.
Denotes significance at the P = 0.05 level with respect to both controls.
Collectively, these results suggest that reduction in dNedd4 levels or interference with its catalytic activity lead to similar muscle innervation defects, which are also similar to those seen with Comm mutants that cannot bind dNedd4 or become ubiquitinated.
DISCUSSION
Earlier work had demonstrated that endocytosis of Comm is a necessary step for the initiation of neuromuscular synaptogenesis (55). Our work here demonstrates that binding to and ubiquitination of Comm by dNedd4 is a key determinant for Comm endocytosis into muscles, permitting initiation and proper synaptogenesis.
Muscle innervation defects caused by overexpression of Comm(2PY→AY) or Comm(10K→R) mutants or reduction of dNedd4 levels/function.
The genetic data presented in this paper demonstrate that overexpression of mutants Comm(2PY→AY) and Comm(10K→R), catalytically inactive dNedd4(C→A), or reduced dNedd4 function due to RNAi or heterozygosity result in similar synaptic phenotypes, with striking defects observed on muscles 12 and 13, where synaptic boutons are easily visible (43, 48, 50). These muscles, as well as muscles 6 and 7, are normally innervated by the SNb nerve branch. In the above mutants, the motoneurons that target muscle 12 make inappropriate branches that innervate muscle 13 or even the distant muscle 6 (Fig. 4 and 7) (see Fig. S3 and S4 in the supplemental material) (data not shown). Other defects include bypass of muscle 13 or growth cones stalling short of target muscle 12 or 13. This indicates that failure to remove Comm from the cell surface due to impaired dNedd4 binding or function (i.e., ubiquitination) can cause defects in synaptogenesis. These aberrant phenotypes were exclusively observed when mutant Comm overexpression or dNedd4-RNAi was driven by 24B-GAL4, which drives expression early in muscle embryogenesis, because overexpression of the Comm mutants by the Mhc-GAL4 driver, which expresses later in development (first- to third-instar larvae), caused no visible innervation defects.
The ISN pathway also showed some abnormalities in flies expressing the Comm mutants or reduced levels/function of dNedd4 (data not shown), but these were rare and more difficult to score. In particular, the innervation of the distal edge of muscle 2 by a neuron at the terminal end of the ISN pathway was abnormal compared to the expected innervation pattern of the terminal ISN arbor (16, 58). These abnormalities, seen less frequently in the ISN than the SNb pathways, are consistent with those seen previously in comm-deficient flies or those lacking the Comm C terminus (55).
The early observation that both Comm lacking its C terminus (including the PY motifs) and comm-null flies show similar synaptogenesis defects prompted the suggestion that Comm controls another (or other) negative mesodermal regulator(s) (55). Since an extensive body of work has demonstrated that Comm collaborates with Robo to control axon midline crossing in the CNS, it is possible that Robo is also involved in regulating muscle synaptogenesis. Indeed, our initial work revealed synaptogenesis defects in robo1/+ or robo2/+ larvae that express half the dose of these Robo proteins. Interestingly, these defects were suppressed upon simultaneously halving Robo and Comm levels (in robo2/+; comm/+ double heterozygous larvae) (see Fig. S5 in the supplemental material), suggesting genetic interactions between these proteins that affect neuromuscular synaptogenesis. Future studies are needed to investigate the exact role of the different Robo proteins in regulating synaptogenesis, including the elucidation of their developmental and spatial pattern of expression and their putative genetic and biochemical interactions with Comm and dNedd4 during muscle development.
Regulation of Comm endocytosis.
Earlier work (55) has demonstrated that the C terminus of Comm is necessary for Comm endocytosis and muscle synaptogenesis and proposed that a YxxL sequence present within the C terminus (Fig. 1A) may be responsible for the effect. The Yxx-hydrophobic motif is a well-characterized sequence known to bind to μ2 of AP-2, a complex involved in clathrin-mediated endocytosis (3, 33). Our results, however, suggest that binding to a ubiquitin ligase (dNedd4) and the ensuing ubiquitination of Comm (also occurring at its C terminus) are likely the major determinants of Comm endocytosis. This conclusion is based on our observation that overexpression in muscles of UAS-comm mutants that lack the dNedd4 binding sites or ubiquitin acceptor sites but retain an intact YxxL motif are not internalized and show defects in muscle innervation (primarily in the SNb pathway) similar to those seen by Wolf et al. (55) when the whole C terminus was deleted. This suggests that like several other transmembrane proteins, cell surface stability of Comm is regulated by ubiquitination. Moreover, monoubiquitination appears sufficient for Comm endocytosis, bypassing the requirement for interaction with and ubiquitination by dNedd4. Future work is needed to determine whether the increased cell surface accumulation of Comm(2PY→AY) or Comm(10K→R) is due to reduced internalization, increased recycling, or both.
Our work here detects the presence of Comm at the plasma membrane and in various endocytic vesicles at different maturation stages, including early/recycling endosomes. Importantly, using antibodies to the ectodomain of Comm, we clearly demonstrate that a substantial amount of WT Comm found in endosomes of S2 cells originated from the plasma membrane, and moreover, we show that WT Comm accumulates at the muscle surface of shibire mutants at the nonpermissive temperature when internalization of PM proteins is blocked. This would argue that unlike the behavior of ectopically expressed Comm in mammalian cells (23) where it is not naturally expressed (Comm is unique to flies), much of the Comm expressed in fly cells/tissues, including muscle, is likely endocytosed from the plasma membrane. This does not preclude the possibility that Comm targeting to the PM can, in addition, be regulated also by sorting from the TGN (23), as seen, for example, in the case of the Gap1 permease in yeast, where Rsp5 (the yeast homologue of dNedd4) and ubiquitination can regulate both Gap1 internalization (9) and its sorting to multivesicular bodies from the TGN (12, 44). Indeed, Drosophila Nedd4 family members were recently demonstrated to regulate both endocytosis and sorting of Notch (40, 54).
While our earlier work demonstrated the involvement of dNedd4 in regulating CNS axon midline crossing by regulating stability of the Comm/Robo complex (32), a recent report by Keleman et al. (24) suggests that dNedd4 and ubiquitination of Comm are not involved in that process. Although the possible reason(s) for the discrepancy in results is not yet known, it is possible that Comm regulation during CNS midline crossing and synaptogenesis is different. The inability of Keleman et al. (24) to detect Comm ubiquitination may be the result of their use of mammalian Cos7 cells, which do not express dNedd4, while we used Drosophila S2 cells that express it endogenously. Of note, dNedd4 possesses a high-affinity WW domain (WW3*) that is required for high-affinity interaction with Comm and likely for its ubiquitination (13, 18).
Loss of dNedd4 binding to Comm and Comm ubiquitination in the muscle reduces viability.
The UAS-comm(2PY→AY) and UAS-comm(10K→R) larvae showed a temperature-dependent reduction in viability, with some surviving at 21 to 25°C but none at 29°C (the GAL4/UAS expression system is temperature sensitive). The cause of death is unknown, but the larvae appear to be sluggish in their locomotion, possibly due to loss of proper muscle innervation. This lethality was “rescued” in the UAS-comm(2PY→AY-monoUb) and UAS-comm(10K→R-monoUb) larvae, in accord with prevention of the synaptogenesis defects in these flies. All the UAS-dNedd4RNAi/24B-GAL4 and the UAS-dNedd4C→A/24B-GAL4 flies were viable at RT and 29°C. This is not surprising given that the RNAi leads to only a partial removal of dNedd4 from the muscle, and likewise, the catalytically inactive dNedd4(C→A) acts in a dominant-negative fashion, which may not completely ablate function of the endogenous dNedd4. In support, dNedd4/+ heterozygotes, expressing half the normal levels of dNedd4, were viable.
Summary.
Comm was previously proposed as a regulator of synaptogenesis that needs to be removed from the muscle cell surface prior to motoneuron innervation (55). Our work here suggests that this removal is achieved by binding of Comm to the ubiquitin ligase dNedd4 and by ubiquitination of Comm to promote its endocytosis. Thus, we conclude that dNedd4 is a positive regulator of synaptogenesis.
Supplementary Material
Acknowledgments
We thank C. Smith and B. Charlton for reagents, G. Tear for Comm cDNA, anti-Comm-ECD antibodies and comm mutant flies, S. Hayashi for dNedd4 mutant flies, J. R. Jacobs for robo1 and robo2 mutant flies, A. Chiba for the 5-GAL4 driver line, H. Bellen for anti-Hrs antibodies, J. Gruenberg for anti-LBPA antibodies, M. Gonzalez-Gaitan for anti-dRab5 antibodies, Y. Yarden for the Ub(4KR) construct, and R. Vale for GFP-LAMP1-expressing S2 cells. The JLA20 monoclonal antibody was from the Developmental Studies Hybridoma, developed with NICHD support and maintained by The University of Iowa.
This work was supported by grants from the Canadian Institute of Health Research (CIHR) (to D.R. and G.B.), NCIC (with funds from the Canadian Cancer Society) (to D.R.), and CIHR Studentship and Fellowship support (to P.H. and Y.P.). D.R. and G.B. were/are recipients of CIHR Investigator Awards and Canada Research Chair Investigator Awards (Tier I).
Footnotes
Published ahead of print on 30 October 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abriel, H., J. Loffing, J. F. Rebhun, J. H. Pratt, L. Schild, J. D. Horisberger, D. Rotin, and O. Staub. 1999. Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle's syndrome. J. Clin. Investig. 103:667-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellen, H. J., and V. Budnik. 2000. The neuromuscular junction, p. 175-200. In W. Sullivan, M. Ashburner, and R. S. Hawley (ed.), Drosophila protocols. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 3.Boll, W., H. Ohno, Z. Songyang, I. Rapoport, L. C. Cantley, J. S. Bonifacino, and T. Kirchhausen. 1996. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 15:5789-5795. [PMC free article] [PubMed] [Google Scholar]
- 4.Brand, A. H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401-415. [DOI] [PubMed] [Google Scholar]
- 5.Chen, H. I., and M. Sudol. 1995. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc. Natl. Acad. Sci. USA 92:7819-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, X., B. Zhang, and J. A. Fischer. 2002. A specific protein substrate for a deubiquitinating enzyme: liquid facets is the substrate of Fat facets. Genes Dev. 16:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemens, J. C., C. A. Worby, N. Simonson-Leff, M. Muda, T. Maehama, B. A. Hemmings, and J. E. Dixon. 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 97:6499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn, R., and L. Hicke. 2001. Multiple roles for Rsp5p-dependent ubiquitination at the internalization step of endocytosis. J. Biol. Chem. 276:25974-81. [DOI] [PubMed] [Google Scholar]
- 9.Galan, J. M., V. Moreau, B. Andre, C. Volland, and R. Haguenauer-Tsapis. 1996. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J. Biol. Chem. 271:10946-52. [DOI] [PubMed] [Google Scholar]
- 10.Georgiou, M., and G. Tear. 2003. The N-terminal and transmembrane domains of Commissureless are necessary for its function and trafficking within neurons. Mech. Dev. 120:1009-1019. [DOI] [PubMed] [Google Scholar]
- 11.Goodman, C. S., and C. Q. Doe. 1993. Embryonic development of the Drosophila central nervous system, p. 1131-1206. In M. Bate and A. Martinez-Arias (ed.), the development of Drosophila melanogaster, vol. 19. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 12.Helliwell, S. B., S. Losko, and C. A. Kaiser. 2001. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol. 153:649-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry, P. C., V. Kanelis, M. C. O'Brien, B. Kim, I. Gautschi, J. Forman-Kay, L. Schild, and D. Rotin. 2003. Affinity and specificity of interactions between Nedd4 isoforms and the epithelial Na+ channel. J. Biol. Chem. 278:20019-28. [DOI] [PubMed] [Google Scholar]
- 14.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 15.Hicke, L., and R. Dunn. 2003. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19:141-172. [DOI] [PubMed] [Google Scholar]
- 16.Hoang, B., and A. Chiba. 2001. Single-cell analysis of Drosophila larval neuromuscular synapses. Dev. Biol. 229:55-70. [DOI] [PubMed] [Google Scholar]
- 17.Johansen, J., M. E. Halpern, and H. Keshishian. 1989. Axonal guidance and the development of muscle fiber-specific innervation in Drosophila embryos. J. Neurosci. 9:4318-4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanelis, V., C. Bruce, N. Skrynnikov, D. Rotin, and J. D. Forman-Kay. 2006. Structure determinants for high affinity binding in a Nedd4 WW3* domain - Comm PY motif complex. Structure 14:543-553. [DOI] [PubMed] [Google Scholar]
- 19.Kanelis, V., D. Rotin, and J. D. Forman-Kay. 2001. Solution structure of a Nedd4 WW domain - ENaC peptide complex. Nat. Struct. Biol. 8:407-412. [DOI] [PubMed] [Google Scholar]
- 20.Kasanov, J., G. Pirozzi, A. J. Uveges, and B. K. Kay. 2001. Characterizing class I WW domains defines key specificity determinants and generates mutant domains with novel specificities. Chem. Biol. 8:231-241. [DOI] [PubMed] [Google Scholar]
- 21.Katz, M., K. Shtiegman, P. Tal-Or, L. Yakir, Y. Mosesson, D. Harari, Y. Machluf, H. Asao, T. Jovin, K. Sugamura, and Y. Yarden. 2002. Ligand-independent degradation of epidermal growth factor receptor involves receptor ubiquitylation and Hgs, an adaptor whose ubiquitin-interacting motif targets ubiquitylation by Nedd4. Traffic 3:740-751. [DOI] [PubMed] [Google Scholar]
- 22.Katzmann, D. J., M. Babst, and S. D. Emr. 2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106:145-155. [DOI] [PubMed] [Google Scholar]
- 23.Keleman, K., S. Rajagopalan, D. Cleppien, D. Teis, K. Paiha, L. A. Huber, G. M. Technau, and B. J. Dickson. 2002. Comm sorts robo to control axon guidance at the Drosophila midline. Cell 110:415-427. [DOI] [PubMed] [Google Scholar]
- 24.Keleman, K., C. Ribeiro, and B. J. Dickson. 2005. Comm function in commissural axon guidance: cell-autonomous sorting of Robo in vivo. Nat. Neurosci. 8:156-163. [DOI] [PubMed] [Google Scholar]
- 25.Keshishian, H., A. Chiba, T. N. Chang, M. S. Halfon, E. W. Harkins, J. Jarecki, L. Wang, M. Anderson, S. Cash, M. E. Halpern, et al. 1993. Cellular mechanisms governing synaptic development in Drosophila melanogaster. J. Neurobiol. 24:757-787. [DOI] [PubMed] [Google Scholar]
- 26.Kikonyogo, A., F. Bouamr, M. L. Vana, Y. Xiang, A. Aiyar, C. Carter, and J. Leis. 2001. Proteins related to the Nedd4 family of ubiquitin protein ligases interact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proc. Natl. Acad. Sci. USA 98:11199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi, T., E. Stang, K. S. Fang, P. de Moerloose, R. G. Parton, and J. Gruenberg. 1998. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature 392:193-197. [DOI] [PubMed] [Google Scholar]
- 28.Leventis, P. A., B. M. Chow, B. A. Stewart, B. Iyengar, A. R. Campos, and G. L. Boulianne. 2001. Drosophila amphiphysin is a post-synaptic protein required for normal locomotion but not endocytosis. Traffic 2:839-850. [DOI] [PubMed] [Google Scholar]
- 29.Lifton, R. P., A. G. Gharavi, and D. S. Geller. 2001. Molecular mechanisms of human hypertension. Cell 104:545-556. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd, T. E., R. Atkinson, M. N. Wu, Y. Zhou, G. Pennetta, and H. J. Bellen. 2002. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108:261-269. [DOI] [PubMed] [Google Scholar]
- 31.Mosesson, Y., K. Shtiegman, M. Katz, Y. Zwang, G. Vereb, J. Szollosi, and Y. Yarden. 2003. Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J. Biol. Chem. 278:21323-6. [DOI] [PubMed] [Google Scholar]
- 32.Myat, A., P. Henry, V. McCabe, L. Flintoft, D. Rotin, and G. Tear. 2002. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the roundabout receptor. Neuron 35:447-459. [DOI] [PubMed] [Google Scholar]
- 33.Owen, D. J., and P. R. Evans. 1998. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282:1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel, N. H. 1994. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol. 44:445-487. [DOI] [PubMed] [Google Scholar]
- 35.Plant, P. J., F. Lafont, S. Lecat, P. Verkade, K. Simons, and D. Rotin. 2000. Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb. J. Cell Biol. 149:1473-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plant, P. J., H. Yeger, O. Staub, P. Howard, and D. Rotin. 1997. The C2 domain of the ubiquitin protein ligase Nedd4 mediates Ca2+-dependent plasma membrane localization. J. Biol. Chem. 272:32329-36. [DOI] [PubMed] [Google Scholar]
- 37.Polo, S., S. Sigismund, M. Faretta, M. Guidi, M. R. Capua, G. Bossi, H. Chen, P. De Camilli, and P. P. Di Fiore. 2002. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416:451-455. [DOI] [PubMed] [Google Scholar]
- 38.Rose, D., and A. Chiba. 2000. Synaptic target recognition at Drosophila neuromuscular junctions. Microsc. Res. Tech. 49:3-13. [DOI] [PubMed] [Google Scholar]
- 39.Rotin, D., O. Staub, and R. Haguenauer-Tsapis. 2000. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr. Biol. 176:1-17. [DOI] [PubMed] [Google Scholar]
- 40.Sakata, T., H. Sakaguchi, L. Tsuda, A. Higashitani, T. Aigaki, K. Matsuno, and S. Hayashi. 2004. Drosophila Nedd4 regulates endocytosis of notch and suppresses its ligand-independent activation. Curr. Biol. 14:2228-2236. [DOI] [PubMed] [Google Scholar]
- 41.Schild, L., Y. Lu, I. Gautschi, E. Schneeberger, R. P. Lifton, and B. C. Rossier. 1996. Identification of a PY motif in the epithelial Na channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. EMBO J. 15:2381-2387. [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid, A., A. Chiba, and C. Q. Doe. 1999. Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development 126:4653-4689. [DOI] [PubMed] [Google Scholar]
- 43.Shishido, E., M. Takeichi, and A. Nose. 1998. Drosophila synapse formation: regulation by transmembrane protein with Leu-rich repeats, CAPRICIOUS. Science 280:2118-2121. [DOI] [PubMed] [Google Scholar]
- 44.Soetens, O., J. O. De Craene, and B. Andre. 2001. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J. Biol. Chem. 276:43949-57. [DOI] [PubMed] [Google Scholar]
- 45.Soubeyran, P., K. Kowanetz, I. Szymkiewicz, W. Y. Langdon, and I. Dikic. 2002. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416:183-187. [DOI] [PubMed] [Google Scholar]
- 46.Staub, O., S. Dho, P. Henry, J. Correa, T. Ishikawa, J. McGlade, and D. Rotin. 1996. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J. 15:2371-2380. [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart, B. A., H. L. Atwood, J. J. Renger, J. Wang, and C. F. Wu. 1994. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A 175:179-191. [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi, H., E. Shishido, M. Takeichi, and A. Nose. 2000. Functional dissection of drosophila capricious: its novel roles in neuronal pathfinding and selective synapse formation. J. Neurobiol. 42:104-116. [DOI] [PubMed] [Google Scholar]
- 49.Tear, G., R. Harris, S. Sutaria, K. Kilomanski, C. S. Goodman, and M. A. Seeger. 1996. Commissureless controls growth cone guidance across the CNS midline in Drosophila and encodes a novel membrane protein. Neuron 16:501-514. [DOI] [PubMed] [Google Scholar]
- 50.Umemiya, T., E. Takasu, M. Takeichi, T. Aigaki, and A. Nose. 2002. Forked end: a novel transmembrane protein involved in neuromuscular specificity in drosophila identified by gain-of-function screening. J. Neurobiol. 51:205-214. [DOI] [PubMed] [Google Scholar]
- 51.Vecchione, A., A. Marchese, P. Henry, D. Rotin, and A. Morrione. 2003. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol. Cell. Biol. 23:3363-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, G., J. M. McCaffery, B. Wendland, S. Dupre, R. Haguenauer-Tsapis, and J. M. Huibregtse. 2001. Localization of the Rsp5p ubiquitin-protein ligase at multiple sites within the endocytic pathway. Mol. Cell. Biol. 21:3564-3575.11313482 [Google Scholar]
- 53.Wang, W., and G. Struhl. 2004. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development 131:5367-5380. [DOI] [PubMed] [Google Scholar]
- 54.Wilkin, M. B., A. M. Carbery, M. Fostier, H. Aslam, S. L. Mazaleyrat, J. Higgs, A. Myat, D. A. Evans, M. Cornell, and M. Baron. 2004. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr. Biol. 14:2237-2244. [DOI] [PubMed] [Google Scholar]
- 55.Wolf, B., M. A. Seeger, and A. Chiba. 1998. Commissureless endocytosis is correlated with initiation of neuromuscular synaptogenesis. Development 125:3853-3863. [DOI] [PubMed] [Google Scholar]
- 56.Wucherpfennig, T., M. Wilsch-Brauninger, and M. Gonzalez-Gaitan. 2003. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 161:609-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yasuda, J., M. Nakao, Y. Kawaoka, and H. Shida. 2003. Nedd4 regulates egress of Ebola virus-like particles from host cells. J. Virol. 77:9987-9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshihara, M., M. B. Rheuben, and Y. Kidokoro. 1997. Transition from growth cone to functional motor nerve terminal in Drosophila embryos. J. Neurosci. 17:8408-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.