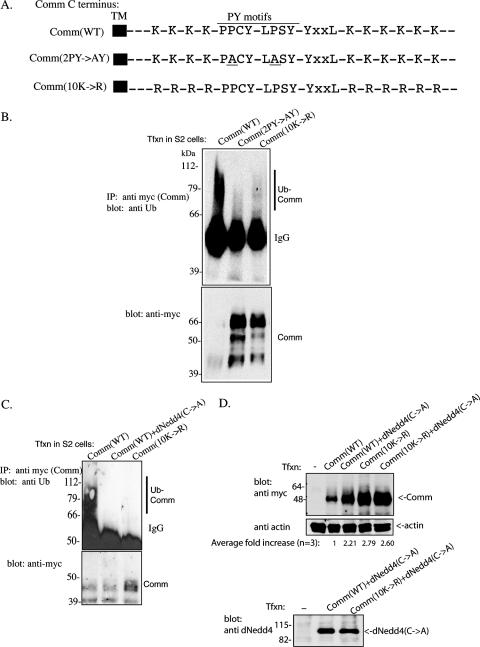
FIG. 1.
Loss of ubiquitination of the Comm(2PY→AY) and Comm(10K→R) mutants. (A) Schematic representation of the C terminus of Comm, showing its 10 Lys residues, two PY motifs, and the YXXL motif. Also shown are the Comm(2PY→AY) and Comm(10K→R) mutants. (Only relevant residues are shown, with spacing between them not to scale.) TM, transmembrane. (B) Drosophila S2 cells were transfected (Tfxn) with myc-tagged wild-type Comm [Comm(WT)], Comm bearing mutations in the two PY motifs to abolish binding to dNedd4 [Comm(2PY→AY], or Comm mutated in all 10 of its cytoplasmic Lys residues [Comm(10K→R] that serve as putative ubiquitin acceptor sites. Comm (WT or mutants) was then immunoprecipitated (IP) from cells, and the IPs were immunoblotted with antiubiquitin (Ub) antibodies to detect ubiquitinated Comm (upper panel). Comm expression levels in the lysates were verified with anti-myc antibodies (lower panel). Note that a substantial fraction of Comm(WT), which is ubiquitinated, disappears (likely degraded), whereas the Comm mutants are stabilized. (C) The same as panel B, only also showing loss of Comm(WT) ubiquitination by endogenous dNedd4 in S2 cells by overexpression of catalytically inactive dNedd4(C→A). Comm protein here (and in other figures) appears as multiple bands, which is the pattern normally observed with Comm, and may represent posttranslational modifications (see reference 23). (D) Increased stability of ubiquitination-impaired Comm [(Comm(10K→R)] or WT Comm coexpressed with catalytically inactive dNedd4(C→A). S2 cells transfected (or not) with myc-tagged Comm(WT) or Comm(10K→R) alone or with Flag-dNedd4(C→A) were lysed, and lysates were analyzed by Western blotting for the amounts of Comm present. Control lower panels depict actin and dNedd4(C→A) expression. The average increase in Comm stability from three independent experiments is indicated as well. IgG, immunoglobulin G.