Figure 1.
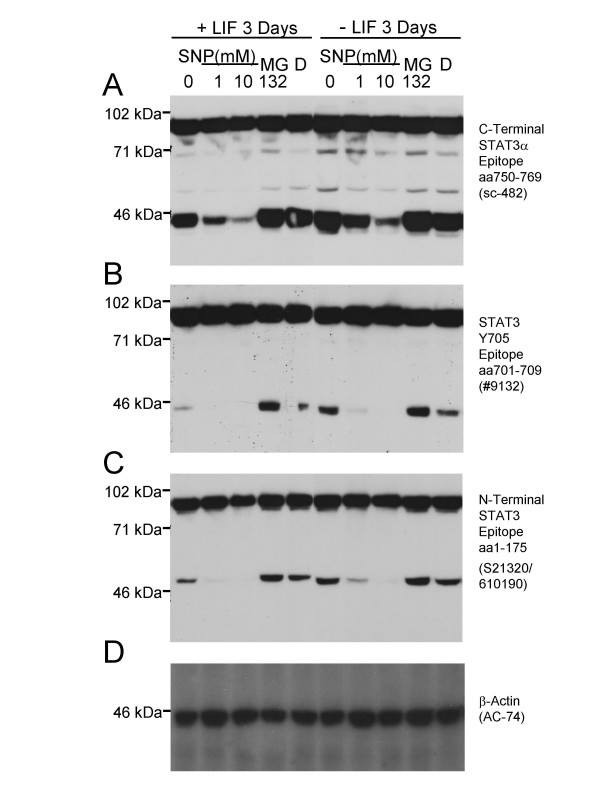
Proteolytic cleavage of STAT3α in HM-1 murine ES cells to 48 kDa and 43 kDa fragments can be inhibited by the NO-donor SNP. A. An antibody (Santa Cruz sc-482) directed against amino acids 750–769 at the carboxy terminus of STAT3α revealed full-length STAT3α and a 43 kDa carboxy terminal cleavage product. 90 μg of protein were loaded per lane. B. Blot stripped and re-probed with an antibody (Cell Signaling Technology #9132) that recognises an epitope around the STAT3 Y705 residue within the carboxy terminal region, again detecting the 43 kDa cleavage fragment. C. Blot stripped and re-probed with an antibody that recognises an epitope within the STAT3 amino terminal amino acid 1–175 region (BD/Transduction Laboratories S21320/610190), detecting full-length STAT3α and a STAT3-derived 48 kDa amino terminal cleavage fragment. Cell culture treatment with the NO-donor (and caspase inhibitor) SNP for 2 hours reduced the level of the 43 kDa and 48 kDa fragments in a dose-dependent manner (lanes 1–3, 6–8 in panels A-C) in HM-1 ES cells cultured in either the presence (+LIF, lanes 1–5) or absence (-LIF, lanes 6y10) of added LIF in the medium. In contrast, the proteasome inhibitor MG132 added to 10 μM in the medium for 2 hours (with DMSO vehicle to a final concentration of 0.2% vol/vol) could not inhibit cleavage (lanes 4, 9 in A-C) relative to untreated cells, or DMSO vehicle alone (D). D. Blot stripped and re-probed with an anti β-Actin monoclonal antibody (Sigma, AC-74) to confirm equal amounts of protein loaded per lane.
