Figure 2.
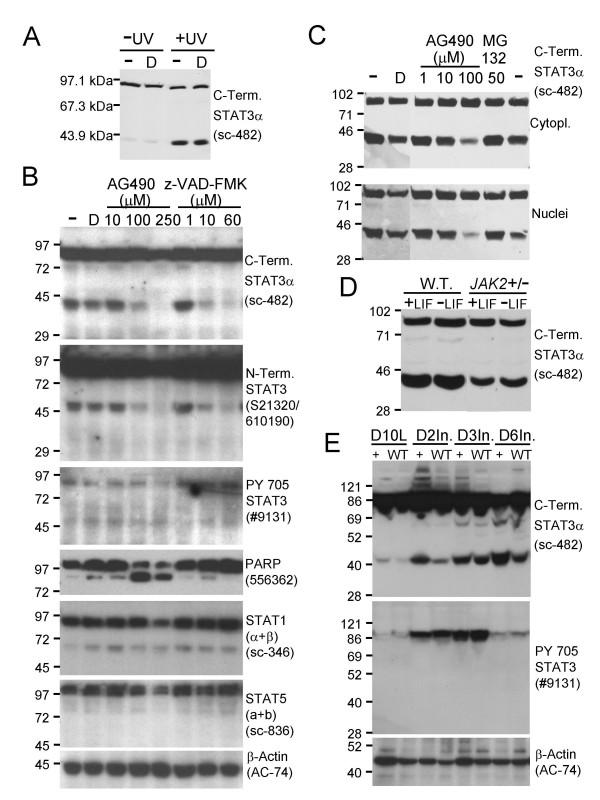
Modulation of STAT3α cleavage by caspase and JAK2 protein tyrosine kinase activity. A. Immunoblot showing a significant increase in STAT3α cleavage following UV irradiation. The Santa Cruz sc-482 antibody (recognising the carboxy terminus of STAT3α) revealed a significant increase in the level of the 43 kDa fragment following UV irradiation (+UV) of HM-1 ES cells (254 nm, 1 mJ/cm2, with harvesting 13 hours later) compared to control un-irradiated (-UV) cells in the presence (D) or absence (-) of 0.5% vol/vol DMSO vehicle, 100 μg of whole cell extract protein loaded per lane. B. Immunoblot demonstrating dose-dependent inhibition of STAT3α cleavage by the pan-caspase inhibitor z-VAD-FMK and the JAK2 tyrosine kinase inhibitor AG490. The 43 and 48 kDa STAT3α fragments were essentially abolished by pretreating HM-1 ES cells with z-VAD-FMK to 60 μM (lanes 1, 2, 6–8, C-term. STAT3α and N-term. STAT3 panels) or AG490 to 250 μM (lanes 1–5, C-term. STAT3α and N-term. STAT3 panels) for 18 hours in the cell culture medium before harvesting. The 250 μM AG490-mediated abolition of STAT3α cleavage correlated with loss of tyrosine phosphorylation of the STAT3α Y705 residue (lane 5, PY 705 STAT3 panel). 75 μg of whole cell extract protein loaded per lane, DMSO added to 0.5% vol/vol in the culture medium in the z-VAD-FMK and AG490 treatments and the DMSO control (D). C. Identical behaviour of HM-1 ES cell cytoplasmic and nuclear extracts following AG490-mediated inhibition of STAT3α cleavage and inability of 50 μM MG132 to inhibit STAT3α cleavage. 100 μg of cytoplasmic or nuclear extract protein loaded per lane, DMSO added to 1% vol/vol in the culture medium for AG490 and MG132 treatments and DMSO controls (D), treatments given for 7 hours prior to cell harvesting. Immunoblots used the Santa Cruz sc-482 antibody. D. Down-regulation of STAT3α cleavage in JAK2 heterozygous knockout (JAK2 +/-) ES cells. Wild type HM-1 and JAK2 +/- ES cells were cultured with (+LIF) or without (-LIF) added LIF for 3 days prior to harvesting. Cytoplasmic extracts (100 μg protein per lane) were immunoblotted with the Santa Cruz sc-482 antibody. E. Immunoblots of RIPA extracts of wild type (W.T.) and SMAD4 transgenic (+) murine mammary glands from day 10 lactation (D10L) and 2 day (D2In.), 3 day (D3In.) and 6 day (D6In.) forced involution timepoints showing the close linkage between appearance of tyrosine 705 phosphorylated STAT3α and the generation of the 43 kDa carboxy terminal STAT3α cleavage product. 20 μg of RIPA whole cell extract protein was loaded per lane.
