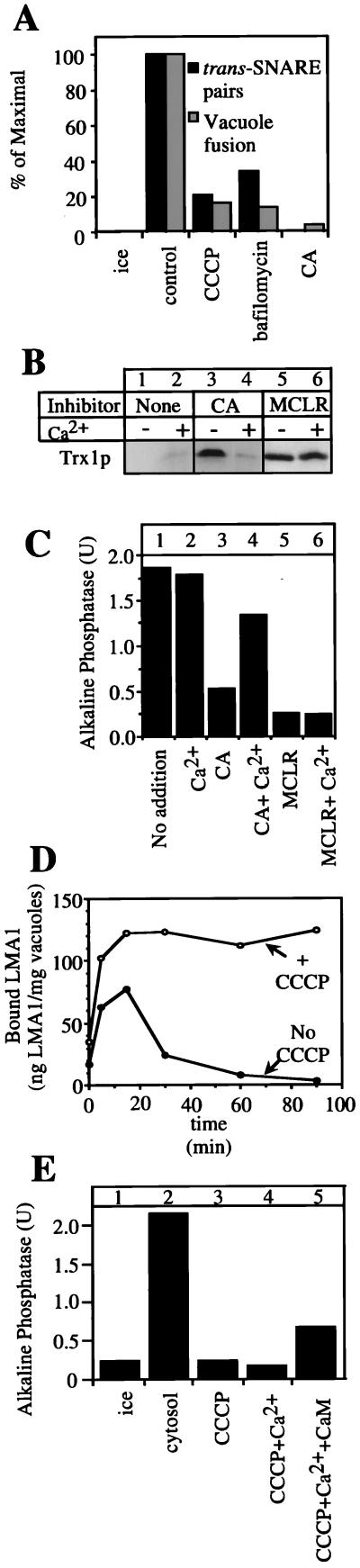
Figure 4.
The membrane potential is required for trans-SNARE pairing and LMA1 release and can be partially restored by Ca2+. (A) Trans-SNARE complex formation requires the vacuolar membrane potential. Vacuoles (100 μg) from the two tester strains (DKY vam3Δ and BJ nyv1Δ) were incubated in the presence of ATP, cytosol, and CCCP (20 μM), Bafilomycin (10 μM), or CA (250 μM) where indicated for 40 min at 27°C and analyzed for trans-SNARE complex (20). An aliquot was further incubated for 140 min to measure fusion. (B and C) Release of LMA1 is blocked by CA and rescued by added Ca2+. Salt-washed vacuoles (equivalent to six standard reactions) were incubated with ATP, Sec18p, LMA1, CoA, and CaM (see Fig. 1) and 20 μM MCLR or 1 mM CA with or without 3 mM Ca2+. After 90 min, vacuoles equivalent to one fusion reaction were transferred to another tube to measure fusion activity (C). The remaining vacuoles were assayed for bound LMA1 (B) as described (18). (D) CCCP blocks LMA1 release. Samples equivalent to five standard reactions were incubated at 25°C with (○) or without (●) CCCP (30 μM). At the indicated times, vacuoles were reisolated (5 min, 8,000 × g, 4°C), resuspended in 300 μl of PS buffer with 1× PIC and 0.5 mM PMSF, reisolated, resuspended in 25 μl of PS buffer, and transferred to a fresh tube. The samples then were analyzed by “High-Tris” SDS/PAGE and immunoblot with anti-Trx1p antibodies (11). Bound LMA1 was quantified by densitometry and normalized to a standard of pure LMA1 (18). (E) CCCP inhibition of vacuole fusion is partially rescued by addition of Ca2+ and CaM. Salt-washed vacuoles were incubated for 100 min in the presence of ATP and cytosol (1 mg/ml) on ice or at 25°C. CCCP (30 μM), Ca2+ (1 mM), and CaM (7.5 μM) were added where indicated. Alkaline phosphatase activity was measured as described in Methods.