Abstract
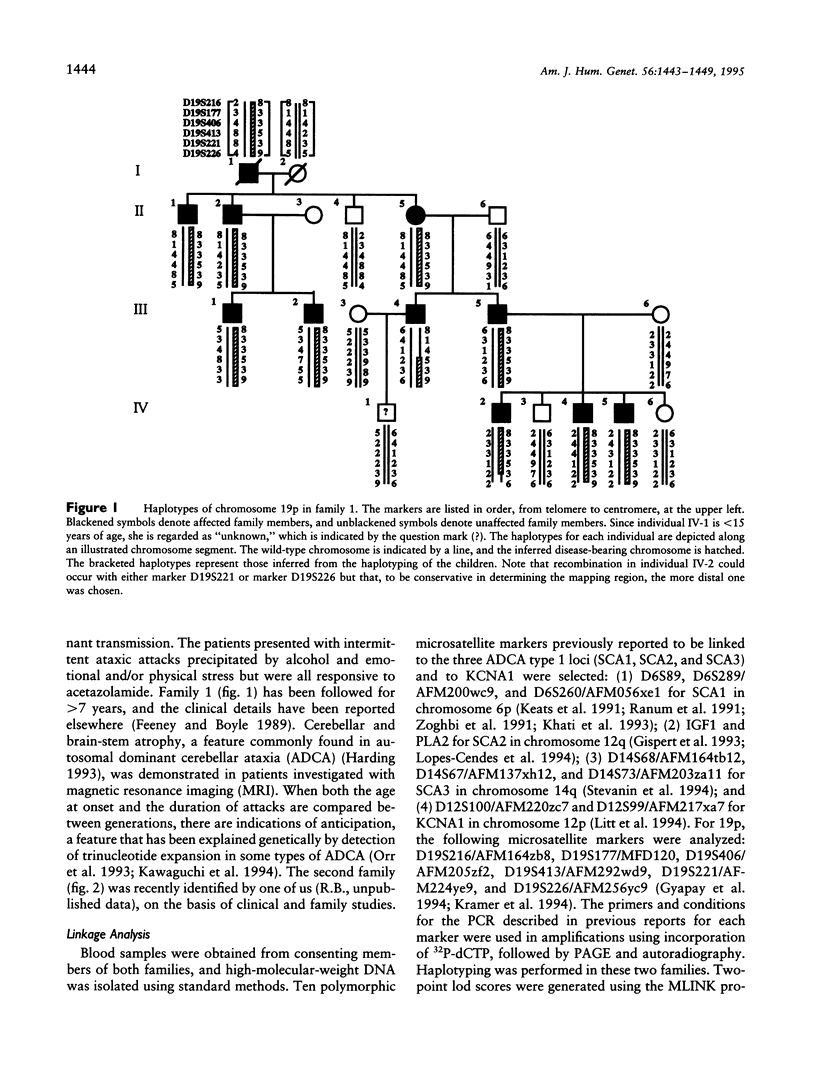
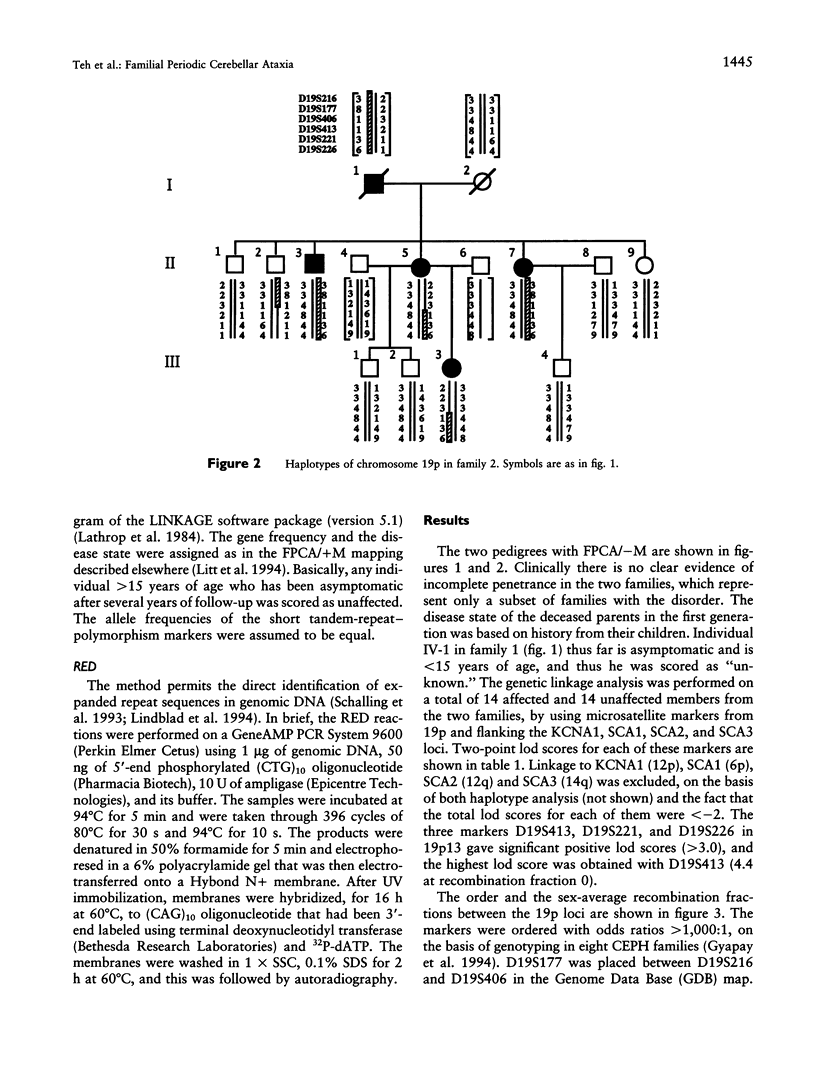
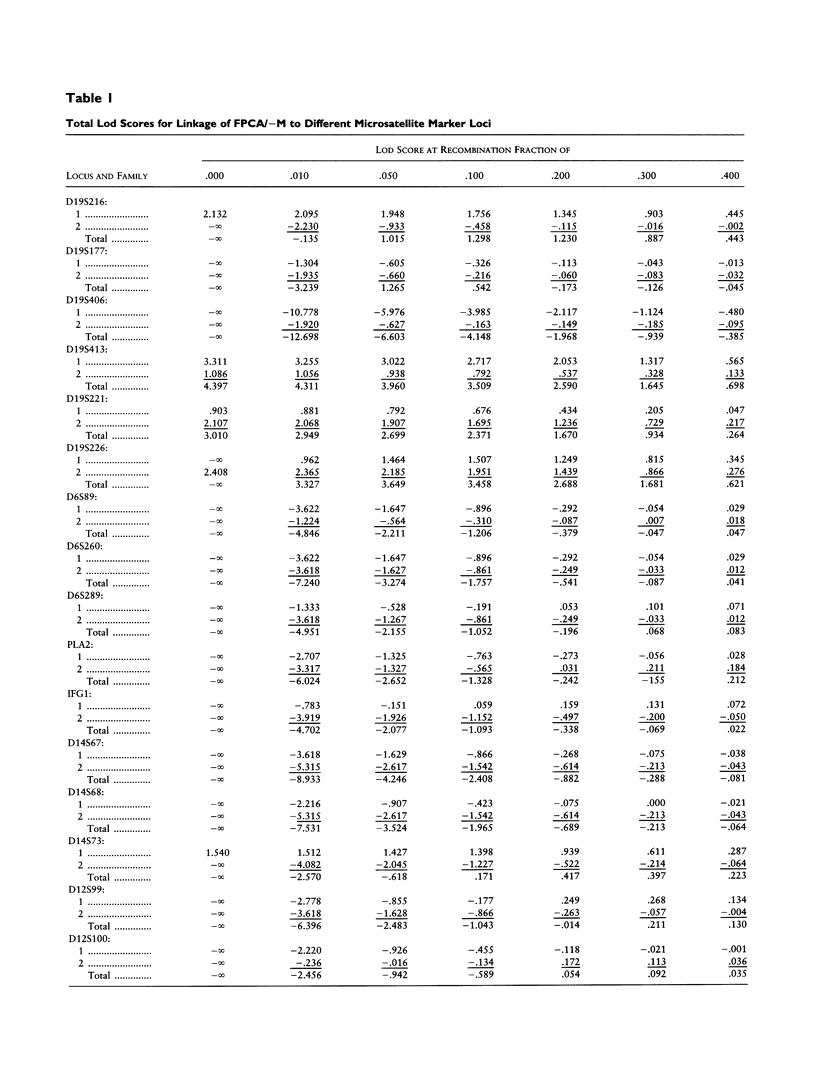
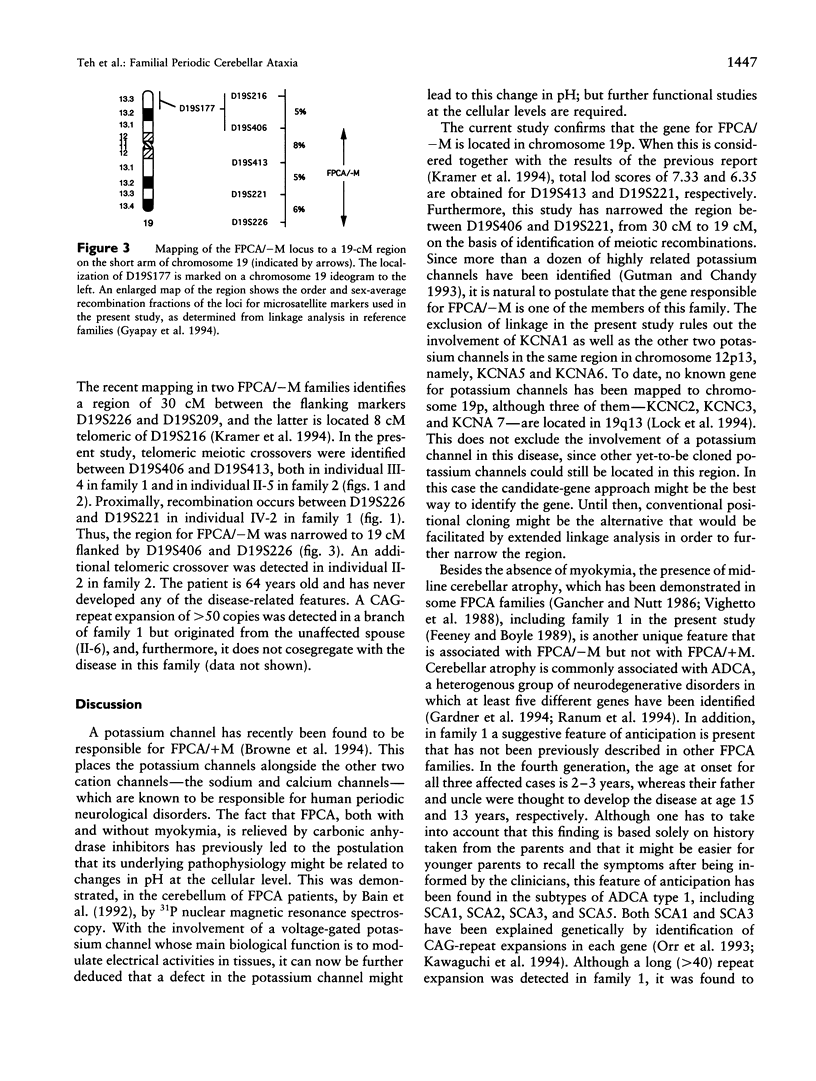
Familial periodic cerebellar ataxia (FPCA) is a heterogeneous group of rare autosomal dominant disorders characterized by episodic cerebellar disturbance. A potassium-channel gene (KCNA1) has been found to be responsible for one of its subgroups, familial periodic cerebellar ataxia with myokymia (FPCA/+M; MIM 160120). A different subgroup that is not associated with myokymia (FPCA/-M; MIM 108500) was recently mapped to chromosome 19p. Here we have performed linkage analysis in two large families with FPCA/-M that also demonstrated neurodegenerative pathology of the cerebellum. Three markers in 19p13 gave significant lod scores (> 3.0), while linkage to KCNA1 and three known loci for spinocerebellar ataxia (SCA1, SCA2, and SCA3) was excluded. The highest lod score was obtained with the marker D19S413 (4.4 at recombination fraction 0), and identification of meiotic recombinants in affected individuals placed the locus between the flanking markers D19S406 and D19S226, narrowing the interval to 19 cM. A CAG trinucleotide-repeat expansion was detected in one family but did not cosegregate with the disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bain P. G., O'Brien M. D., Keevil S. F., Porter D. A. Familial periodic cerebellar ataxia: a problem of cerebellar intracellular pH homeostasis. Ann Neurol. 1992 Feb;31(2):147–154. doi: 10.1002/ana.410310205. [DOI] [PubMed] [Google Scholar]
- Browne D. L., Gancher S. T., Nutt J. G., Brunt E. R., Smith E. A., Kramer P., Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994 Oct;8(2):136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- Brunt E. R., van Weerden T. W. Familial paroxysmal kinesigenic ataxia and continuous myokymia. Brain. 1990 Oct;113(Pt 5):1361–1382. doi: 10.1093/brain/113.5.1361. [DOI] [PubMed] [Google Scholar]
- Feeney G. F., Boyle R. S. Paroxysmal cerebellar ataxia. Aust N Z J Med. 1989 Apr;19(2):113–117. doi: 10.1111/j.1445-5994.1989.tb00216.x. [DOI] [PubMed] [Google Scholar]
- Friedman J. H., Hollmann P. A. Acetazolamide responsive hereditary paroxysmal ataxia. Mov Disord. 1987;2(1):67–72. doi: 10.1002/mds.870020110. [DOI] [PubMed] [Google Scholar]
- Gancher S. T., Nutt J. G. Autosomal dominant episodic ataxia: a heterogeneous syndrome. Mov Disord. 1986;1(4):239–253. doi: 10.1002/mds.870010404. [DOI] [PubMed] [Google Scholar]
- Gispert S., Twells R., Orozco G., Brice A., Weber J., Heredero L., Scheufler K., Riley B., Allotey R., Nothers C. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23-24.1. Nat Genet. 1993 Jul;4(3):295–299. doi: 10.1038/ng0793-295. [DOI] [PubMed] [Google Scholar]
- Griggs R. C., Moxley R. T., 3rd, Lafrance R. A., McQuillen J. Hereditary paroxysmal ataxia: response to acetazolamide. Neurology. 1978 Dec;28(12):1259–1264. doi: 10.1212/wnl.28.12.1259. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Joutel A., Bousser M. G., Biousse V., Labauge P., Chabriat H., Nibbio A., Maciazek J., Meyer B., Bach M. A., Weissenbach J. A gene for familial hemiplegic migraine maps to chromosome 19. Nat Genet. 1993 Sep;5(1):40–45. doi: 10.1038/ng0993-40. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Okamoto T., Taniwaki M., Aizawa M., Inoue M., Katayama S., Kawakami H., Nakamura S., Nishimura M., Akiguchi I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994 Nov;8(3):221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Keats B. J., Pollack M. S., McCall A., Wilensky M. A., Ward L. J., Lu M., Zoghbi H. Y. Tight linkage of the gene for spinocerebellar ataxia to D6S89 on the short arm of chromosome 6 in a kindred for which close linkage to both HLA and F13A1 is excluded. Am J Hum Genet. 1991 Nov;49(5):972–977. [PMC free article] [PubMed] [Google Scholar]
- Khati C., Stevanin G., Durr A., Chneiweiss H., Belal S., Seck A., Cann H., Brice A., Agid Y. Genetic heterogeneity of autosomal dominant cerebellar ataxia type 1: clinical and genetic analysis of 10 French families. Neurology. 1993 Jun;43(6):1131–1137. doi: 10.1212/wnl.43.6.1131. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad K., Zander C., Schalling M., Hudson T. Growing triplet repeats. Nat Genet. 1994 Jun;7(2):124–124. doi: 10.1038/ng0694-124. [DOI] [PubMed] [Google Scholar]
- Litt M., Kramer P., Browne D., Gancher S., Brunt E. R., Root D., Phromchotikul T., Dubay C. J., Nutt J. A gene for episodic ataxia/myokymia maps to chromosome 12p13. Am J Hum Genet. 1994 Oct;55(4):702–709. [PMC free article] [PubMed] [Google Scholar]
- Lock L. F., Gilbert D. J., Street V. A., Migeon M. B., Jenkins N. A., Copeland N. G., Tempel B. L. Voltage-gated potassium channel genes are clustered in paralogous regions of the mouse genome. Genomics. 1994 Apr;20(3):354–362. doi: 10.1006/geno.1994.1188. [DOI] [PubMed] [Google Scholar]
- Lopes-Cendes I., Andermann E., Attig E., Cendes F., Bosch S., Wagner M., Gerstenbrand F., Andermann F., Rouleau G. A. Confirmation of the SCA-2 locus as an alternative locus for dominantly inherited spinocerebellar ataxias and refinement of the candidate region. Am J Hum Genet. 1994 May;54(5):774–781. [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Ranum L. P., Duvick L. A., Rich S. S., Schut L. J., Litt M., Orr H. T. Localization of the autosomal dominant HLA-linked spinocerebellar ataxia (SCA1) locus, in two kindreds, within an 8-cM subregion of chromosome 6p. Am J Hum Genet. 1991 Jul;49(1):31–41. [PMC free article] [PubMed] [Google Scholar]
- Ranum L. P., Schut L. J., Lundgren J. K., Orr H. T., Livingston D. M. Spinocerebellar ataxia type 5 in a family descended from the grandparents of President Lincoln maps to chromosome 11. Nat Genet. 1994 Nov;8(3):280–284. doi: 10.1038/ng1194-280. [DOI] [PubMed] [Google Scholar]
- Schalling M., Hudson T. J., Buetow K. H., Housman D. E. Direct detection of novel expanded trinucleotide repeats in the human genome. Nat Genet. 1993 Jun;4(2):135–139. doi: 10.1038/ng0693-135. [DOI] [PubMed] [Google Scholar]
- Stevanin G., Le Guern E., Ravisé N., Chneiweiss H., Dürr A., Cancel G., Vignal A., Boch A. L., Ruberg M., Penet C. A third locus for autosomal dominant cerebellar ataxia type I maps to chromosome 14q24.3-qter: evidence for the existence of a fourth locus. Am J Hum Genet. 1994 Jan;54(1):11–20. [PMC free article] [PubMed] [Google Scholar]
- Vighetto A., Froment J. C., Trillet M., Aimard G. Magnetic resonance imaging in familial paroxysmal ataxia. Arch Neurol. 1988 May;45(5):547–549. doi: 10.1001/archneur.1988.00520290083018. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y., Jodice C., Sandkuijl L. A., Kwiatkowski T. J., Jr, McCall A. E., Huntoon S. A., Lulli P., Spadaro M., Litt M., Cann H. M. The gene for autosomal dominant spinocerebellar ataxia (SCA1) maps telomeric to the HLA complex and is closely linked to the D6S89 locus in three large kindreds. Am J Hum Genet. 1991 Jul;49(1):23–30. [PMC free article] [PubMed] [Google Scholar]


