Abstract
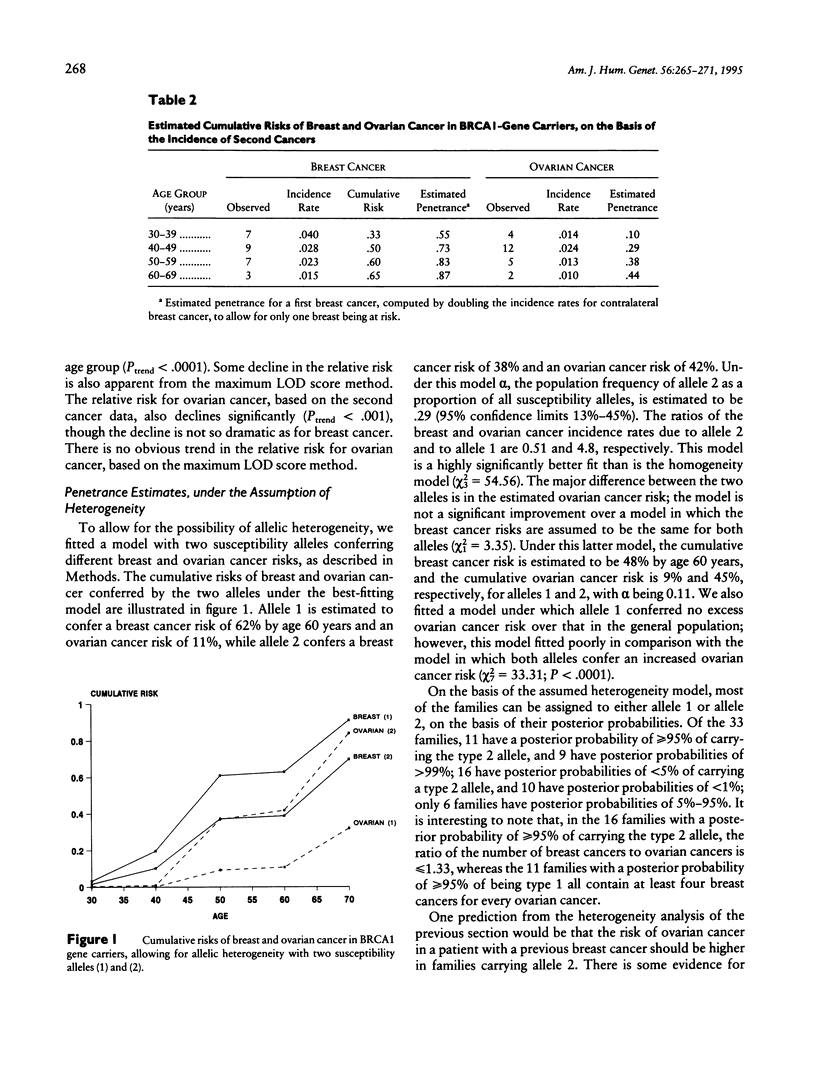
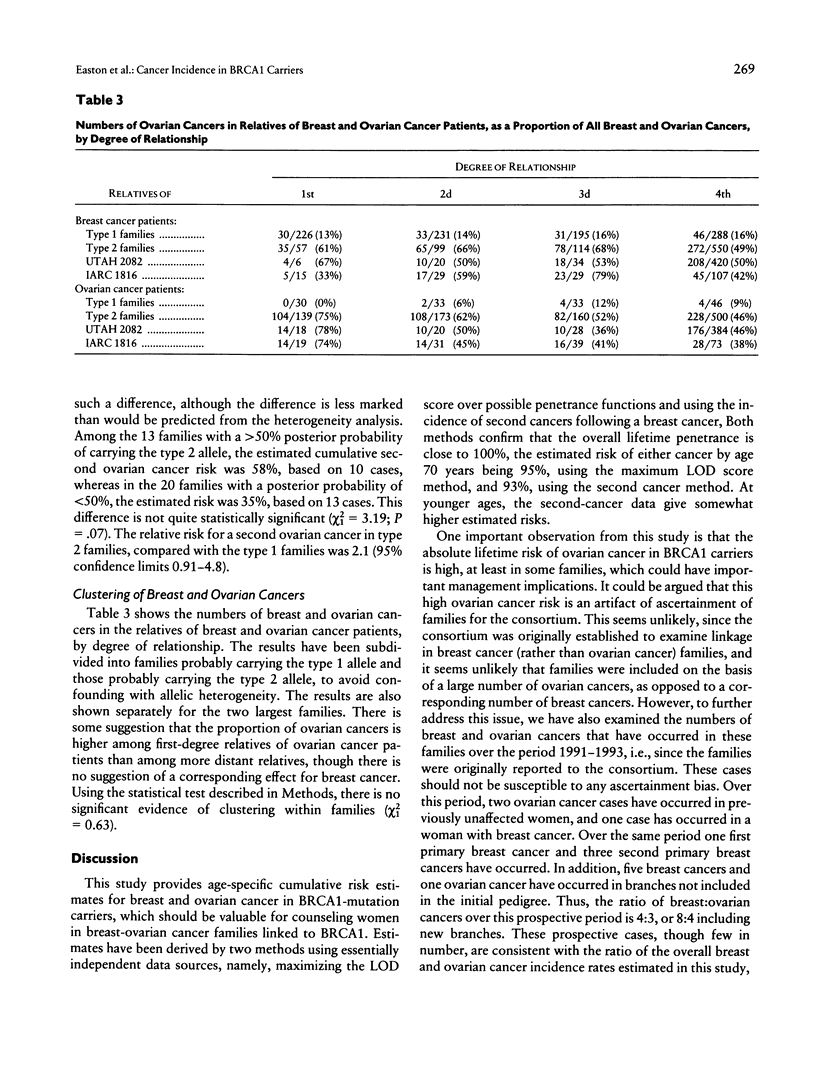
Dominant predisposition to early-onset breast cancer and/or ovarian cancer in many families is known to be the result of germ-line mutations in a gene on chromosome 17q, known as BRCA1. In this paper we use data from families with evidence of linkage to BRCA1 to estimate the age-specific risks of breast and ovarian cancer in BRCA1-mutation carriers and to examine the variation in risk between and within families. Under the assumption of no heterogeneity of risk between families, BRCA1 is estimated to confer a breast cancer risk of 54% by age 60 years (95% confidence interval [CI] 27%-71%) and an ovarian cancer risk of 30% by age 60 years (95% CI 8%-47%). Similar lifetime-risk estimates are obtained by examining the risks of contralateral breast cancer and of ovarian cancer, in breast cancer cases in linked families. However, there is significant evidence of heterogeneity of risk between families; a much better fit to the data is obtained by assuming two BRCA1 alleles, one conferring a breast cancer risk of 62% and an ovarian cancer risk of 11% by age 60 years, the other conferring a breast cancer risk of 39% and an ovarian cancer risk of 42%, with the first allele representing 71% of all mutations (95% CI 55%-87%). There is no evidence of clustering of breast and ovarian cancer cases within families.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowcock A. M., Anderson L. A., Friedman L. S., Black D. M., Osborne-Lawrence S., Rowell S. E., Hall J. M., Solomon E., King M. C. THRA1 and D17S183 flank an interval of < 4 cM for the breast-ovarian cancer gene (BRCA1) on chromosome 17q21. Am J Hum Genet. 1993 Apr;52(4):718–722. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. S., Boehnke M., Frank T. S., Kiousis S., Xu J., Guo S. W., Hauser E. R., Norum R. A., Helmbold E. A., Markel D. S. BRCA1 maps proximal to D17S579 on chromosome 17q21 by genetic analysis. Am J Hum Genet. 1993 Apr;52(4):792–798. [PMC free article] [PubMed] [Google Scholar]
- Easton D. F., Bishop D. T., Ford D., Crockford G. P. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993 Apr;52(4):678–701. [PMC free article] [PubMed] [Google Scholar]
- Feunteun J., Narod S. A., Lynch H. T., Watson P., Conway T., Lynch J., Parboosingh J., O'Connell P., White R., Lenoir G. M. A breast-ovarian cancer susceptibility gene maps to chromosome 17q21. Am J Hum Genet. 1993 Apr;52(4):736–742. [PMC free article] [PubMed] [Google Scholar]
- Ford D., Easton D. F., Bishop D. T., Narod S. A., Goldgar D. E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994 Mar 19;343(8899):692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Goldgar D. E., Fields P., Lewis C. M., Tran T. D., Cannon-Albright L. A., Ward J. H., Swensen J., Skolnick M. H. A large kindred with 17q-linked breast and ovarian cancer: genetic, phenotypic, and genealogical analysis. J Natl Cancer Inst. 1994 Feb 2;86(3):200–209. doi: 10.1093/jnci/86.3.200. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Friedman L., Guenther C., Lee M. K., Weber J. L., Black D. M., King M. C. Closing in on a breast cancer gene on chromosome 17q. Am J Hum Genet. 1992 Jun;50(6):1235–1242. [PMC free article] [PubMed] [Google Scholar]
- Hall J. M., Lee M. K., Newman B., Morrow J. E., Anderson L. A., Huey B., King M. C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Narod S. A., Feunteun J., Lynch H. T., Watson P., Conway T., Lynch J., Lenoir G. M. Familial breast-ovarian cancer locus on chromosome 17q12-q23. Lancet. 1991 Jul 13;338(8759):82–83. doi: 10.1016/0140-6736(91)90076-2. [DOI] [PubMed] [Google Scholar]
- Narod S. A., Ford D., Devilee P., Barkardottir R. B., Lynch H. T., Smith S. A., Ponder B. A., Weber B. L., Garber J. E., Birch J. M. An evaluation of genetic heterogeneity in 145 breast-ovarian cancer families. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995 Jan;56(1):254–264. [PMC free article] [PubMed] [Google Scholar]
- Risch N. Segregation analysis incorporating linkage markers. I. Single-locus models with an application to type I diabetes. Am J Hum Genet. 1984 Mar;36(2):363–386. [PMC free article] [PubMed] [Google Scholar]
- Steichen-Gersdorf E., Gallion H. H., Ford D., Girodet C., Easton D. F., DiCioccio R. A., Evans G., Ponder M. A., Pye C., Mazoyer S. Familial site-specific ovarian cancer is linked to BRCA1 on 17q12-21. Am J Hum Genet. 1994 Nov;55(5):870–875. [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., Kwitek A. E., May P. E., Wallace M. R., Collins F. S., Ledbetter D. H. Dinucleotide repeat polymorphisms at the D17S250 and D17S261 loci. Nucleic Acids Res. 1990 Aug 11;18(15):4640–4640. [PMC free article] [PubMed] [Google Scholar]


