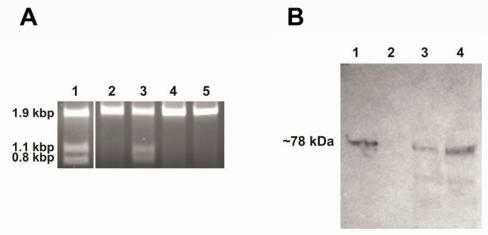
Figure 3.
Demonstration of endonuclease activity and immunoblot assay. Panel A: Digestion of the S. cerevisiae V-ATPase catalytic subunit gene (vma1) without intein. The reactions were performed for one hour at 37oC in PI-SceI reaction buffers in presence of 2.5 mM metal ions. The target site is present in a 1.9kb DNA sequence and after cleavage two fragments of 1.1 kb and 0.8 kb should be observed. Lane 1: Digestion with purified PI-SceI homing endonuclease using the reaction buffer in presence of 2.5mM MgCl2. Lane 2, 3, 4, and 5: Digestion with purified PI-SceI117GFP using the reaction buffer in presence of 2.5mM MgCl2, MnCl2, ZnCl2, or CaCl2 respectively. As shown, the GFP-fusion PI-SceI homing endonuclease only cleaves the target site in presence of MnCl2. Panel B: Immunoblot assay using commercial anti-Sce VMA1 intein antibodies. Lane 1: Purified PI-SceI117GFP protein (positive control). Lane 2: Protein extract from XL1B (DE3) without plasmid (negative control). Lane 3 and 4: Protein extracts from XL1B (DE3) with plasmids expressing PI-SceI 117GFP3.9kb and PI-SceI 117GFP3kb respectively. Expression was performed in minimal media as described in the experimental procedure.