Figure 3.
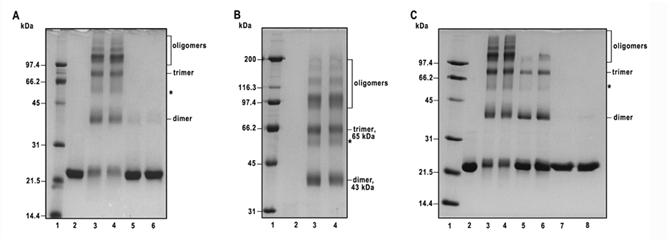
Probing of the oligomeric structure of recombinant bovine factor B by chemical cross-linking. A, The cross-linking pattern of recombinant factor B obtained using bis (sulfosuccinimidyl) suberate (BS, spacer arm 11. 4 Å) and disulfosuccinimidyl tartrate (sulfo-DST, spacer arm 6.4 Å). Lane 2, control, no cross-linker added; lanes 3 and 4, cross-linking of factor B (1.5 mg protein per ml) with 1 mM BS for 5 min and 10 min at room temperature, respectively; lanes 5 and 6, cross-linking of factor B (1.5 mg protein per ml) with 1 mM sulfo-DST for 5 and 10 min, respectively. The cross-linking reactions were quenched with 100 mM ammonium acetate, and the samples were analyzed by 12% SDS-PAGE under reducing conditions, followed by staining with Coomassie brilliant blue. Each lane was loaded with 4 μg of factor B. B, Samples identical to those shown in lanes 2-4 of A were separated on 9 % SDS-PAGE under reducing conditions. In the experimental conditions employed, the non-cross-linked factor B ∼23 kDa monomeric species ran off the gel and is not visible in the lane 2. Lanes 3 and 4 contain recombinant factor B cross-linked with 1 mM BS for 5 and 10 min, respectively. Each lane was loaded with 6.5 μg of factor B. C, The effect of factor B protein concentration on cross-linking pattern with 1 mM BS. Lane 2, control, no cross-linker added; lanes 3 and 4, factor B (2 mg protein per ml) cross-linked with 1 mM BS for 5 and 10 min, respectively; lanes 5 and 6, factor B (0.2 mg protein per ml) cross-linked with 0.1 mM BS for 5 and 10 min, respectively; lanes 7 and 8, factor B (0.02 mg protein per ml) cross-linked with 10 μM BS for 5 and 10 min, respectively. Each lane was loaded with 5.2 μg of factor B. Shown is a 12% SDS-PAGE stained with Coomassie brilliant blue. Lane 1 in A and C, and lane 1 in B contain low and high molecular weight standards, respectively, and their Mr values (in kDa) are shown on the left.
