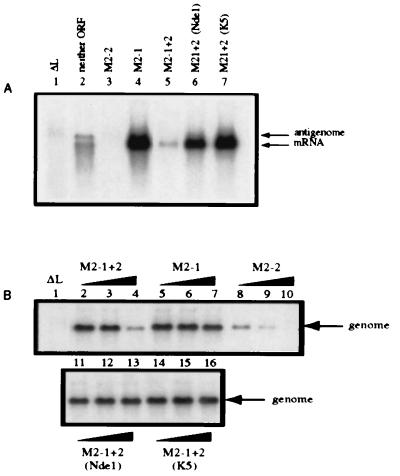
Figure 2.
The NdeI and K5 mutations each ablate the inhibitory function of M2–2 in a reconstituted minireplicon system. (A) HEp-2 cells were simultaneously infected with vTF7–3 (five plaque-forming units per cell) and transfected with plasmid encoding the negative-sense C2 minigenome cDNA (200 ng) and support plasmids (N, 400 ng; P, 200 ng; L, 100 ng per well of a six-well dish) and supplemented with pTM constructs (80 ng) expressing neither M2 ORF (lane 2), M2–2 (lane 3), M2–1 (lane 4), M2–1 + 2 (lane 5), or the M2–1 + 2 containing the NdeI (lane 6) or K5 (lane 7) mutations. Lane 1 contains RNA from a reaction that lacked L and is a negative control. Cells were exposed to 2 μg actinomycin D per milliliter from 24–26 hr after infection (21). At 48 hr after infection, total intracellular RNA was isolated and electrophoresed on formaldehyde gels for Northern blot analysis (16). Blots were hybridized to a negative-sense CAT-specific riboprobe to detect both mRNA and miniantigenome. (B) HEp-2 cells were transfected as described above with plasmid encoding positive-sense C4 miniantigenome complemented by the N, P, and L plasmids as in A. The transfection mixtures were supplemented with increasing amounts (0.008, 0.04, and 0.2 times the relative molar ratio of transfected pTM-N) of pTM constructs expressing M2–1 + 2 (lanes 2, 3, and 4), M2–1 (lanes 5, 6, and 7), M2–2 (lanes 8, 9, and 10) or M2–1 + 2 containing the NdeI (lanes 11, 12, and 13) or K5 (lanes 14, 15, and 16) mutation. Total intracellular RNA was analyzed by Northern blots hybridized with a positive-sense CAT-specific riboprobe to detect genomic RNA.