Fig. 4.
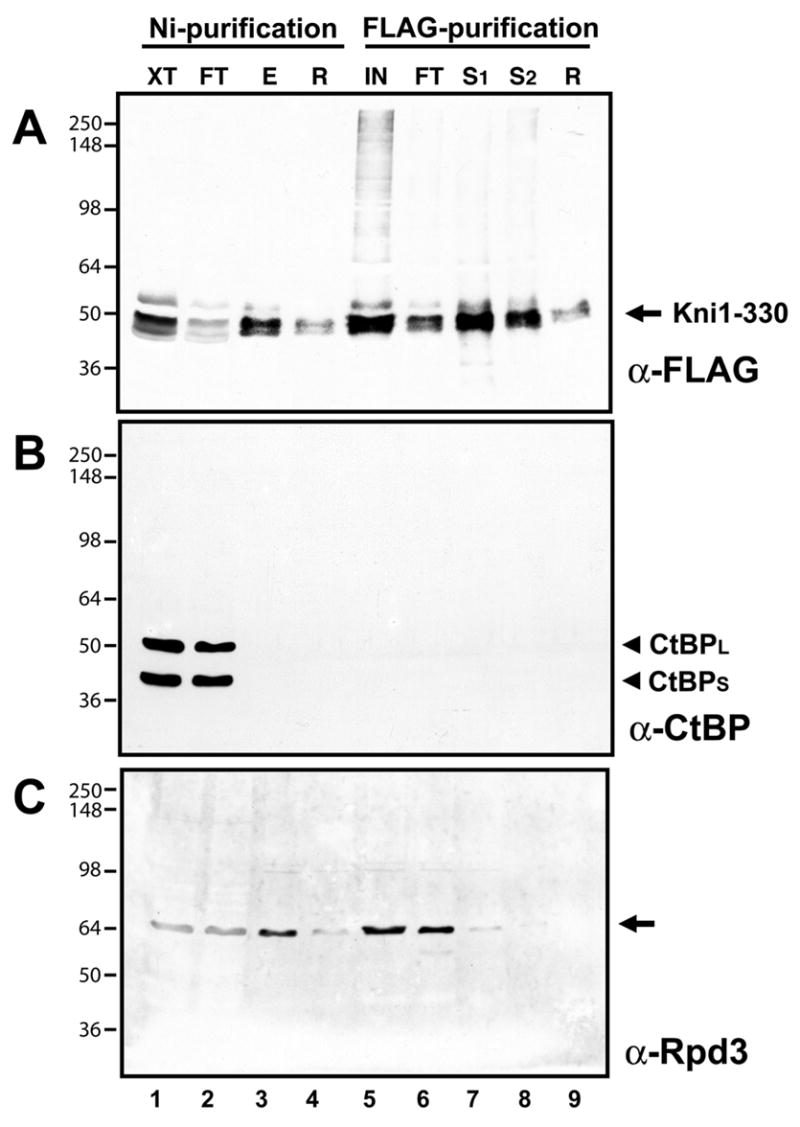
Rpd3 does not copurify through two step affinity purification with N-terminal, CtBP-independent repression domain of Knirps. Double affinity purification was conducted with extracts from embryos expressing the his6- and FLAG-tagged Knirps 1–330 protein, which is active for repression (9). A, Western blot showing Ni-NTA and anti-FLAG purification of recombinant Knirps 1–330 protein. Crude lysate (XT, lane 1, 0.16% of the total), material not bound (FT, lane 2, 0.16% of the total), imidazole eluate from Ni-NTA resin (E, lane 3, 0.3% of the total), material remaining on Ni-NTA, not eluted by imidazole (R, lane 4, 0.2% of the total), pooled eluates from Ni-NTA (IN, lane 5, 20% of the total), material not bound by anti-FLAG protein G sepharose beads (FT, lane 6, 20% of the total), material eluted from antibody beads with Sarkosyl (S1–2, lanes 7–8, 20% of the total), and material remaining on beads (R, lane 9, 20% of the total). B, CtBP protein that is present in the crude extract (lane 1) does not copurify with Knirps, and is instead detected only in the unbound fraction (lane 2). Western blot performed using anti-CtBP antibodies. C, Western blot using anti-Rpd3 antibodies shows presence of Rpd3 protein in fractions. Some of the Rpd3 protein present in the initial fraction does bind to the Ni-NTA column and co-purifies with Knirps in this first step (lane 3), but this protein does not copurify with Knirps 1–330 in the second step, and is found mainly in the unbound fraction (lane 6).
