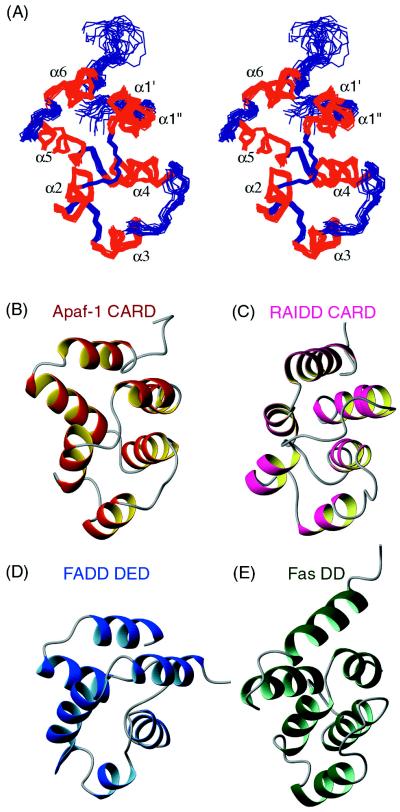
Figure 1.
Solution structure of Apaf-1 CARD resembles other homophilic interacting motifs in apoptosis. (A) Stereoview of the backbone atoms (N, Cα, C′) of the 15 superimposed NMR-derived structures of Apaf-1 CARD. The helices are numbered α1–α6 accordingly. (B–E) Ribbon diagrams of Apaf-1 CARD shown in red (B), RAIDD CARD in pink (C), FADD DED in dark blue (D), and Fas DD in green (E), illustrating the conserved six-helix bundle motif as well as variations of helix orientations among different domains. The coordinates of RAIDD CARD, FADD DED, and Fas DD were obtained from the Protein Data Bank (accession codes 3crd, 1a1w, and 1ddf).