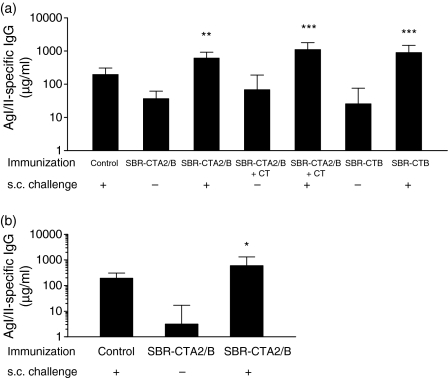
Figure 2.
Surface antigen AgI/II-specific immunoglobulin G (IgG) in serum following intragastric (i.g.) immunization with or without systemic challenge. Groups (n = 4–6) were immunized on days 0, 10 and 20 with a genetically coupled protein composed of the saliva-binding region (SBR) of the Streptococcus mutans surface antigen AgI/II and the non-toxic A2 and B subunits of cholera toxin (CT) (SBR-CTA2/B), recombinant SBR admixed with CT (SBR-CTA2/B + CT) or SBR chemically coupled to the recombinant CT B subunit (SBR-CTB), and challenged with AgI/II subcutaneously (s.c.) 2 weeks(a)or 5 months(b)later. Control animals received AgI/II s.c. only. Samples were collected 7 days following the final i.g. or s.c. immunization. Results are represented as the geometric mean and standard deviation. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with the corresponding group that was not challenged.