Abstract
Although the role of natural killer (NK) cells in mycobacterial infections is unclear, it has been postulated that they contribute to protective immunity through the production of interferon (IFN)-γ. In this study, we evaluate the effect of interleukin (IL)-10, IL-15 and IL-18 on NK lytic activity through the expression of CD16, CD11a and CD69 molecules and the induction of IFN-γ production in patients with tuberculosis (TB) and healthy individuals (N). Our results showed an impairment of NK lytic activity and a gradual down-regulation of costimulatory and adhesion molecules on NK cells which were dependent on the severity of the disease. NK lytic activity was increased by exogenous IL-15 and IL-18 in both TB and N, and by neutralization of endogenous IL-10 only in TB; IL-15 and IL-18 increased CD69 receptor expression, while anti-IL-10 up-regulated CD16 and CD11a expression in TB. Mycobacterium tuberculosis reduced the number of intracellular adhesion molecule (ICAM)-1+ CD14+ cells, but in the presence of IL-15, IL-18 and anti-IL-10 its expression was up-regulated. In cells from TB patients, the observed effects of IL-15 and IL-18 on NK function were not dependent on IL-10 modulation of the surface expression of activator/adhesion molecules. In the absence of monocytes, IL-10 activated NK cells, suggesting an indirect effect on their function. Furthermore, in TB patients the depletion of monocytes increased the production of IFN-γ by NK cells. Therefore, monocytes from TB patients regulated the NK function involving IL-10 which, through an indirect mechanism, led to the down-regulation of costimulatory/adhesion molecules and/or IFN-γ production.
Keywords: natural killer cells, cytotoxicity, receptors, monocytes, tuberculosis
Introduction
A protective immune response against Mycobacterium tuberculosis depends on interferon (IFN)-γ production by CD4+ and CD8+ T cells.1 However, the early production of IFN-γ by cells of the innate immune response at inflammatory sites can regulate innate resistance by activating phagocytic cells and priming antigen-presenting cells (APCs) for interleukin (IL)-12 production, thus shaping adaptive immunity towards the T helper type 1 (Th1) response necessary for elimination of many intracellular pathogens. Natural killer (NK) cells are critical components of the innate immune response that lack expression of the T-cell receptor (TCR)–CD3 complex and surface immunoglobulins but express CD56 antigen. NK cells are characterized by potent cytotoxic activity against tumours, virus-infected cells and intracellular parasites.2 Resting NK cells circulate in the blood and, once activated, they are able to migrate to and infiltrate sites of infection where target cells are localized. The interaction of NK with target cells is mediated by various cell surface molecules, some involved in cell adhesion, others activating the NK cytolytic programme, and others inhibiting this activation by negative signalling.2 The best studied activation receptor is the FcγRIII (CD16) molecule through which NK cells mediate antibody-dependent cellular cytotoxicity (ADCC) against target cells coated with immunoglobulin G (IgG).2 Although not restricted to NK cells, integrins [CD11a/CD18, lymphocyte function-associated antigen (LFA)-2 and LFA-3] which bind to intracellular adhesion molecule (ICAM)-1 (or CD54), ICAM-2 and ICAM-3 ligands have also been implicated in NK cell adhesion to target cells, degranulation and cytokine production.3,4 Also, the CD69 molecule, which is rapidly acquired following activation and belongs to the family of C-type lectin receptors bearing strong similarity to the NK receptor CD94,5,6 has been implicated in the cytotoxic activity and costimulation of cytokine production of activated NK cells.7 It is unclear which mechanisms contribute to the priming phase of NK cell activation, but these cells can be rapidly activated in the periphery by chemokines in conjunction with IL-2 and macrophage or dendritic cell (DC)-derived cytokines such as IL-12, IL-15 and IL-18.8,9
Bacterial products activate macrophages to produce IL-12, IL-15 and IL-18, playing a central role in the type 1 cytokine response and NK activity.8,10,11 NK cell cytokine production is induced by IL-12 in synergy with IL-15 and IL-18,8,12,13 which stimulates IFN-γ production by NK cells via different intracellular pathways.13–15 Considering that protection against intracellular pathogens is critically dependent on the function of NK cells at early stages of the immune response and on type-1 cells at later stages, the aim of the present study was to investigate the costimulatory molecules involved in M. tuberculosis-induced NK function through IFN-γ production in patients with tuberculosis. The role of IL-15 and IL-18 was also investigated. Our results show that, in patients with tuberculosis, monocytes regulate NK activity.
Materials and methods
Patients
A total of 42 male patients with pulmonary tuberculosis (TB) were included in the study. Patients were diagnosed by the presence of recent clinical symptoms of TB, a positive sputum smear test for acid-fast bacilli confirmed by a positive culture of TB bacilli and an abnormal chest radiography. Informed consent was obtained from patients according to the Hospital Francisco J. Muñiz Ethics Committee. All patients had active TB and were under multidrug treatment at the time of study (0–15 days). Pulmonary disease was classified according to the extent and type of X-ray findings as mild (M) or advanced (A) TB according to the American Tuberculosis Society criteria. Patients with diabetes, chronic renal failure or malignant diseases, as well as those who tested positive for human immunodeficiency virus (HIV) or other concurrent infectious diseases, were excluded. Patients were classified into two groups: (i) patients with moderate tuberculosis (M-TB; n = 22; age range 25–55 years) and (ii) patients with advanced tuberculosis (A-TB; n = 20, age range 22–60 years). Fourteen healthy individuals (age range 25–60 years) were included as controls.
Mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by Ficoll-Hypaque gradient centrifugation16 and then suspended in RPMI 1640 (Gibco Laboratory, New York, NY) containing gentamycin (85 µg/ml) and 15% heat-inactivated fetal calf serum (FCS) (Gibco) (complete medium).
Monocyte-depleted PBMC
In order to obtain monocyte-depleted cell suspensions, PBMC (5 × 106 cells/well) were incubated on the bottoms of 24-well Falcon plates for 2 hr at 37° to allow cells to adhere to the plastic (85–95% of monocytes). After removal of adherent cells, non-adherent lymphocytes were washed and suspended in complete medium. CD14+ monocyte-depleted cell suspensions were obtained by magnetic methods by treatment of PBMC with anti-CD14 monoclonal antibody (Immunotech, Marseille, France) for 30 min at 4° and then with goat anti-mouse IgG-coated beads (Dynal, Oslo, Norway). Monocyte-depleted cell suspensions (both adherent-depleted and CD14-depleted populations) were suspended in complete medium, ensuring that the proportion of each lymphocyte subset was the same as in total cultured PBMC in order to allow comparison of NK lytic activities. The proportion of contaminated monocytes in cell suspensions depleted by adherence was 1–2% and that in cell suspensions depleted by magnetic methods was 0·5–0·6%.
Antigen
The γ-irradiated M. tuberculosis H37-Rv strain used in this study was kindly provided by Dr J. T. Belisle (Colorado University, Denver, CO). Mycobacteria were suspended in phosphate-buffered saline (PBS) free of pyrogen, sonicated and adjusted at a concentration of 1 × 108 bacteria/ml.
PBMC culture
PBMC (2 × 106 cells/ml) or monocyte-depleted PBMC (1·9 × 106 cells/ml) were cultured in Falcon 2063 tubes (Becton Dickinson, Lincoln, NJ) at 37° in a humidified 5% CO2 atmosphere, in complete medium with or without M. tuberculosis (1 × 106 bacteria/ml), IL-15 (10 ng/ml), IL-18 (20 ng/ml), IL-10 (10 ng/ml) or a monoclonal antibody specific for human IL-10 (10 ng/ml) (Peprotech, Rocky Hill, NJ). After 24 hr of incubation, M. tuberculosis-stimulated and/or cytokine-treated and control cells were washed three times with RPMI 1640, suspended in complete medium (2 × 106 cells/ml) and tested for cytotoxic activity, expression of surface antigens and IFN-γ production by flow cytometry. An isotype-matched non-relevant control IgG1/κ antibody for anti-IL10 was also tested and was found to have no significant effect on cytotoxicity, cytokine production or surface antigen expression.
Cytotoxic assay
Freshly isolated (ex vivo) and cultured PBMC or monocyte-depleted PBMC were added in triplicate at different effector to target cell ratios, in a final volume of 200 µl, to 51Cr-labelled K562 target cells (5 × 103 cells seeded into each well of 96-well microtitre plates; Coming incorporated (NY) Corning, USA). Plates were centrifuged at 50 g for 5 min and incubated at 37° in 5% CO2 for 4 hr. After centrifugation at 200 g for 5 min, 100 µl of supernatant was removed from each well. The radioactivity of supernatants and pellets was measured in a gamma counter. Results were expressed as percentage of cytotoxicity (% Cx):
 |
where c.p.m. is counts per minute. The radioactivity released from target cells incubated with complete medium alone was considered to represent spontaneous release. It ranged from 5 to 10%.
Results expressed in lytic units (LU) for 107 effector cells were calculated by defining 1 LU of cytotoxic activity as the number of effector cells required to lyse 30% of K562 target cells. These cell numbers were readily obtained from the dose–response curves.
Immunofluorescence analysis
Expression of CD16, CD11a and CD69 on CD3– CD56+ lymphocytes and ICAM-1 on CD14+ cells
In order to evaluate the expression of CD16, CD11a and CD69 antigens on CD3– CD56+ NK cells, freshly isolated (ex vivo), cultured or monocyte-depleted PBMC were incubated for 30 min at 4° with the following anti-human antibodies: Cy5PE-CD3 (eBioscience, San Diego, CA), PE-CD56 (Immunotech), FICT-CD16 (Ancell, Bayport, MN), FICT-CD11a (Caltag, Burlingam, CA) or FICT-CD69 (Ancell). ICAM-1 was evaluated in control and M. tuberculosis-stimulated PBMC with or without IL-15, IL-18 and anti-IL-10 for 18 hr and stained with PE-CD54 (eBioscience) and FITC-CD14 (Ancell). PECy5-, FITC- or PE-labelled-isotype matched antibodies were also tested to evaluate non-specific staining. Stained cells were analysed by flow cytometry by acquiring 10 000–20 000 events, and gates were set to forward and side-scatter to exclude cell debris and apoptotic cells. Results are expressed as the percentage of positive cells or mean fluorescence intensity (MFI).
Measurement of IFN-γ+ cells
To determine the expression of intracytoplasmic IFN-γ in control and M. tuberculosis-stimulated CD3– CD56+ cells, PBMC or monocyte-depleted PBMC were cultured for 24 hr with or without M. tuberculosis and/or IL-15 or IL-18. Brefreldin A (5 µg/ml; Sigma, St Louis, MO) was added for the final 4 hr to block IFN-γ secretion. Cells were washed, and 5 × 105 cells suspended in 100 µl of PBS-azide were incubated with anti-CD3 and anti-CD56 MoAb (Ancell) for 15 min at room temperature. Thereafter, the cells were fixed according to the manufacturer's instructions (IntraPrep™ permeabilization reagent; Immunotech), washed with PBS supplemented with 1% FCS and 0·01% azide (PBS-FCS-azide) and suspended in 100 µl of PBS-FCS-azide. Fluorescein-conjugated antibody for IFN-γ (Caltag) was added together with 100 µl of permeabilizing solution (IntraPrep™) and incubated for 30 min at 4°, washed with PBS-FCS-azide, suspended in Isoflow™ (Becton Dickinson) and analysed by flow cytometry. 20 000 events were acquired for each sample and results are expressed as the percentage of IFN-γ-positive cells in the CD3– CD56+ population.
Statistics
Comparisons of TB and N were performed using Student's t-test. Cytoxicity values or flow cytometry data obtained from the different subsets of effector cells of each individual were compared using the Wilcoxon signed rank test.
Results
CD3− CD56+ cells in patients with tuberculosis showed reduced cytotoxicity against K562 target cells
Human NK cells are defined phenotypically by their expression of CD56 and their lack of expression of CD3, and two subsets of NK cells with different levels of CD56 expression have recently been identified.17 The majority of cytotoxic human NK cells have low expression of CD56 (CD56dim) and high expression of Fcγ receptor III (CD16+), and have potent cytotoxic activity against tumour cells and cells infected with virus or bacteria. A minor population of NK cells, expressing the CD56bright CD16dim or CD56brightCD16– phenotype, produce cytokines following activation of monocytes. Therefore, we analysed the proportions of CD3– CD56+ CD16+/– cells and of these two subsets of cells (CD3– CD56dim CD16+ and CD3– CD56bright CD16dim/–) in peripheral blood mononuclear cells (PBMC) and 24-hr-cultured PBMC from M-TB and A-TB patients and healthy (N) controls by flow cytometry. As shown in Table 1, a larger proportion of CD3– CD56+ CD16+/– cells was observed in recently isolated (ex vivo) and M. tuberculosis-stimulated PBMC from TB patients, which also showed an increased proportion of the CD3– CD56dim CD16+ subset relative to N controls. Moreover, M. tuberculosis stimulation did not modify the proportion of either subset in TB patients or N controls. Thus, M. tuberculosis stimulation did not modify the percentage of CD3– CD56dim CD16+ or CD3–CD56bright CD16– cells found in PBMC from TB patients and N controls.
Table 1.
Percentage and lytic activity of CD3– CD56+ cells from patients with tuberculosis (TB) and healthy controls (N)
| M-TB | A-TB | N | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ex vivo | Control | M.tb | Ex vivo | Control | M.tb | Ex vivo | Control | M.tb | |
| % total NK cells | 20 ± 3* | 21 ± 2* | 20 ± 2* | 17 ± 3* | 17 ± 3 | 18 ± 3* | 7 ± 2 | 8 ± 2 | 8 ± 3 |
| % CD56dim CD16+ | 16 ± 1 | 15 ± 2* | 15 ± 3* | 18 ± 2* | 18 ± 3* | 18 ± 2* | 8 ± 2 | 8 ± 2 | 8 ± 2 |
| % CD56bright CD16– | 0·6 ± 0·2 | 0·7 ± 0·1 | 0·6 ± 0·2 | 1·0 ± 0·5 | 1·3 ± 0·5 | 1·0 ± 0·5 | 0·7 ± 0·1 | 0·6 ± 0·1 | 0·6 ± 0·1 |
| NK Cx (LU30) | 43 ± 21 | 45 ± 31 | 58 ± 4*1 | 24 ± 22,3 | 25 ± 32,3 | 29 ± 31,3 | 57 ± 2 | 58 ± 4 | 71 ± 5* |
Peripheral blood mononuclear cells (PBMC) were isolated from 22 patients with moderate tuberculosis (M-TB) and 20 patients with advanced tuberculosis (A-TB) and 14 healthy controls (N). The percentages of CD3– CD56+ CD16+/–, CD56dim CD16+ and CD56bright CD16– cells from ex vivo and 24-hr-cultured PBMC in complete medium (control) or Mycobacterium tuberculosis (M.tb) were determined. The cytotoxic assay was performed employing K562 as target cells at different effector to target cell ratios, and lytic units (LU) for each individual were calculated. Results are expressed as mean ± standard error of the mean (SEM). Statistic differences: M. tuberculosis-stimulated PBMC versus control PBMC:
P < 0·05; patients versus N:
P < 0·05
P < 0·02; A-TB versus M-TB:
P < 0·05.
NK, natural killer; Cx, cytotoxicity.
To assess whether the larger number of CD56dimCD16+ cells could be related to higher cytotoxic activity, ex vivo, control and M. tuberculosis-stimulated PBMC were tested for their ability to lyse the NK-sensitive cell line K562. As shown in Table 1, the lowest lytic activity was observed in cells from A-TB patients, while in M-TB patients cytotoxicity was slightly diminished with respect to the N controls. In addition, in TB patients, NK lytic activity did not correlate with the days of treatment (data not shown). M. tuberculosis induced an increase in lytic activity in M-TB patients and N controls but not in A-TB patients. Thus, the high proportion of CD56dim CD16+ cells observed in TB patients was associated with a progressive loss of NK lytic activity as the disease became more severe. Furthermore, NK cells from A-TB patients were not able to respond to M. tuberculosis stimulation as did cells from M-TB and N individuals.
Low cytotoxic activity in A-TB patients was related to low expression of adhesion/activation molecules on NK cells
The activation of NK cells required for target cell lysis is mediated by a balance of inhibitory and activatory NK receptors as well as various adhesion and costimulatory molecules.2 Among these molecules, the CD16 antigen is one of the most extensively studied activating receptors in the signalling of NK cells. Signalling via CD16 results in the production of cytokines and several chemokines, and causes degranulation of NK cells.18 In addition, it is known that conjugate formation between NK and target cells is a prerequisite for target cell lysis and is critically dependent on engagement of the β2 integrin LFA-1 to its ligands on APCs.19 Although constitutively expressed on few cell types, CD69 is rapidly acquired following in vitro activation.6 Thus, we determined by flow cytometry the percentages and expression levels of CD16, CD11a and CD69 molecules on CD3– CD56+ cells stimulated or not stimulated with M. tuberculosis for 18 hr.
As shown in Table 2, no differences in CD56 expression between TB patients and N controls were observed, but the expression of the CD16 molecule was reduced in CD3– CD56+ cells from A-TB patients compared with those from M-TB patients and N controls. Furthermore, M. tuberculosis induced a slight down-regulation in CD16 expression in A-TB patients. The CD11a molecule was expressed in NK cells from patients and N controls (100% in all groups), although at low levels in cells from A-TB patients, and was not modified by M. tuberculosis stimulation. However, similar percentages of CD69+ NK cells were found in control PBMC cultures from TB patients and N individuals [% CD69+ cells in CD3– CD56+ cells; mean ± standard error of the mean (SEM): M-TB: 28 ± 4; A-TB: 18 ± 4; N: 24 ± 3]. However, M. tuberculosis induced the activation of the CD69 receptor in NK cells from M-TB and N controls (% CD69+ NK cells; mean ± SEM: M-TB: 46 ± 5, P < 0·05; N: 67 ± 7, P < 0·05) but not in A-TB patients (28 ± 5). Furthermore, the lowest expression of CD69 was observed in control and M. tuberculosis-stimulated NK cells from A-TB patients (Table 2). Taken together, these results show a gradual down-regulation of costimulatory and adhesion molecules on NK cells correlated with the severity of the disease, which could explain the impairment in lytic activity.
Table 2.
Expression of CD56, CD16, CD11a and CD69 molecules on CD3– CD56dim natural killer (NK) cells from patients with tuberculosis (TB) and healthy controls (N)
| CD56 | CD16 | CD11a | CD69 | |||||
|---|---|---|---|---|---|---|---|---|
| PBMC from | Control | M.tb | Control | M.tb | Control | M.tb | Control | M.tb |
| M-TB | 305 ± 25 | 299 ± 31 | 265 ± 34 | 291 ± 41 | 271 ± 17 | 250 ± 48 | 134 ± 26* | 189 ± 26 |
| A-TB | 292 ± 29 | 281 ± 18 | 132 ± 22*2 | 110 ± 23*2 | 139 ± 38*1 | 109 ± 26**1 | 75 ± 6***2 | 75 ± 5***3 |
| N | 282 ± 37 | 258 ± 40 | 254 ± 62 | 268 ± 62 | 256 ± 22 | 236 ± 17 | 189 ± 20 | 238 ± 21 |
Peripheral blood mononuclear cells (PBMC) from 22 patients with moderate tuberculosis (M-TB), 20 patients with advanced tuberculosis (A-TB) and 14 healthy controls (N) were cultured without or with Mycobacterium tuberculosis (control and M.tb, respectively) for 24 hr and the expression of CD56, CD16, CD11a and CD69 molecules was determined. Results are expressed as mean fluorescence intensity (MIF), and the mean ± standard error of the mean (SEM) is shown. Statistical differences: patients versus N:
P < 0·05
P < 0·02
P < 0·005; A-TB versus M-TB:
P < 0·05
P < 0·02
P < 0·005.
IL-18 and IL-15 enhanced NK cell activity and up-regulated the expression of CD69, CD16 and CD11a molecules on NK cells from TB patients
It is well known that NK cells constitutively express several monocyte cytokine receptors, including those for IL-10, IL-12, IL-15 and IL-18, and thus receive some of the earliest activation signals from monocytes during the innate immune response. As IL-15 and IL-18 induce NK lysis and IFN-γ production by NK cells,8,13,14 we analysed their role in the regulation of NK cell activation in TB patients. Thus, IL-15 and IL-18 were added to PBMC stimulated or not stimulated with M. tuberculosis for 18 hr and their lytic activity was tested. As shown in Table 3, addition of IL-15 or IL-18 enhanced NK activity in control or M. tuberculosis-stimulated PBMC from M-TB patients and N controls. However, IL-15 but not IL-18 increased NK activity in A-TB patients.
Table 3.
Effect of interleukin (IL)-15 and IL-18 on CD16, CD69 and CD11a molecules and natural killer (NK) cell lysis
| MFI | |||||
|---|---|---|---|---|---|
| PBMC from | PBMC incubated with | % cytotoxicity | % CD69+ NK cells | CD16 | CD11a |
| M-TB | – | 23 ± 3 | 32 ± 5 | 234 ± 45 | 245 ± 53 |
| IL-15 | 38 ± 2* | 43 ± 6 | 235 ± 53 | 245 ± 37 | |
| IL-18 | 35 ± 3 | 43 ± 6 | 238 ± 45 | 251 ± 49 | |
| M.tb | 33 ± 2 | 48 ± 5 | 255 ± 43 | 265 ± 37 | |
| M.tb + IL-15 | 48 ± 3** | 77 ± 3* | 281 ± 34 | 307 ± 43 | |
| M.tb + IL-18 | 42 ± 2* | 65 ± 3* | 284 ± 36 | 285 ± 43 | |
| – | 12 ± 2 | 17 ± 3 | 141 ± 27 | 140 ± 26 | |
| A-TB | IL-15 | 22 ± 3* | 25 ± 4 | 133 ± 32 | 165 ± 45 |
| IL-18 | 17 ± 2 | 19 ± 4 | 135 ± 29 | 168 ± 36 | |
| M.tb | 16 ± 2 | 20 ± 3 | 134 ± 20 | 109 ± 26 | |
| M.tb + IL-15 | 32 ± 2* | 83 ± 4* | 195 ± 21* | 190 ± 35* | |
| M.tb + IL-18 | 23 ± 3 | 45 ± 3* | 163 ± 34 | 168 ± 26* | |
| – | 31 ± 3 | 22 ± 2 | 254 ± 56 | 256 ± 52 | |
| N | IL-15 | 41 ± 3* | 31 ± 3 | 247 ± 48 | 260 ± 48 |
| IL-18 | 46 ± 4* | 25 ± 4 | 250 ± 53 | 250 ± 47 | |
| M.tb | 46 ± 4 | 65 ± 9 | 268 ± 63 | 246 ± 28 | |
| M.tb + IL-15 | 62 ± 4* | 74 ± 3 | 269 ± 49 | 250 ± 43 | |
| M.tb + IL-18 | 59 ± 4* | 70 ± 5 | 273 ± 61 | 259 ± 41 | |
Peripheral blood mononuclear cells (PBMC) from 14 patients with moderate tuberculosis (M-TB), 16 patients with advanced tuberculosis (A-TB) and 12 healthy controls (N) were incubated with or without Mycobacterium tuberculosis (M.tb), in the presence or absence of interleukin (IL)-15 or IL-18, for 24 hr and then used as effector cells (E) against K562 target cells (T) (E:T ratio 40 : 1) and the percentage of cytotoxicity was determined. CD16 and CD11a expression [mean fluorescence intensity (MIF)] and the percentage of CD69+ on CD3– CD56+ cells were also evaluated by flow cytometry. Results are expressed as mean ± standard error of the mean (SEM). Statistical differences: PBMC + IL-15 or IL-18 versus PBMC without cytokines:
P < 0·05
P < 0·02.
Given that IL-15 and IL-18 increased NK activity, we investigated whether the observed effect was also related to the modulation of CD69, CD16 and CD11a molecules on CD3– CD56+ cells. Therefore, PBMC were stimulated with M. tuberculosis in the presence or absence of IL-15 or IL-18 for 18 hr, and cell surface expression of CD69, CD16 and CD11a on CD3– CD56+ cells was examined by flow cytometry. As shown in Table 3, the proportion of CD69+ NK cells in TB patients was increased by M. tuberculosis stimulation in the presence of IL-15 and IL-18. In addition, in cells from A-TB patients, IL-15 modified CD16 and CD11a expression, while IL-18 up-regulated only the expression of the CD11a molecule. However, neither IL-15 nor IL-18 modified these molecules in N control cells. Taken together, these results suggest that these cytokines modulate the activation of NK cells in TB patients.
IL-10 down-regulated NK lytic activity and the expression of CD16, CD11a and CD69
It has been demonstrated that lipopolysaccharide (LPS)-stimulated monocytes can down-regulate the proliferation of CD3– CD56+ NK cells through IL-10 secretion.20 In addition, increased numbers of CD14+ IL-10+ cells in response to M. tuberculosis stimulation and low expression of costimulatory molecules on monocytes from A-TB patients have been demonstrated.21 Thus, to investigate the role of IL-10 in NK activation, PBMC were incubated with IL-10 (TB patients or N controls) or anti-IL-10 (TB patients) for 18 hr and their lytic activity was tested. As shown in Table 4, exogenous IL-10 inhibited control and M. tuberculosis-induced NK activity in N controls. In contrast, the neutralization of endogenous IL-10 enhanced NK cytotoxicity in TB patients, suggesting a role of IL-10 in NK function.
Table 4.
Interleukin (IL)-10 modulated natural killer (NK) cell cytotoxicity
| % cytotoxicity | |||
|---|---|---|---|
| PBMC treated with | M-TB | A-TB | N |
| – | 23 ± 3 | 12 ± 2 | 31 ± 3 |
| IL-10 | 19 ± 2 | 10 ± 2 | 19 ± 5* |
| anti-IL-10 | 27 ± 5 | 16 ± 2 | – |
| M.tb | 33 ± 2 | 16 ± 2 | 46 ± 4 |
| M.tb + IL-10 | 22 ± 4 | 15 ± 3 | 30 ± 4* |
| M.tb + anti-IL-10 | 46 ± 1* | 42 ± 3* | – |
Peripheral blood mononuclear cells (PBMC) from 10 patients with moderate tuberculosis (M-TB), 12 patients with advanced tuberculosis (A-TB) and seven healthy controls (N) were incubated with or without Mycobacterium tuberculosis (M.tb) in the presence or absence of interleukin (IL)-10 or anti-IL-10 for 18 hr and then used as effector cells against K562 target cells (E:T ratio 40 : 1). Results are expressed as the percentage of cytotoxicity [mean ± standard error of the mean (SEM)]. Statistical differences: IL-10/anti-IL-10-treated cells versus untreated cells:
P < 0·05.
In order to investigate whether the low expression of CD69, CD16 and CD11a molecules on NK cells could be ascribed to IL-10 production by cells from A-TB patients, anti-IL-10 was added to PBMC cultures from four A-TB patients and, after 18 hr, the cells were tested for surface expression of those molecules. As shown in Table 5, the neutralization of endogenous IL-10 enhanced the expression of CD16 and CD11a molecules, whereas the number of CD69+ cells increased in M. tuberculosis-stimulated NK cells. In the presence of M. tuberculosis, the addition of IL-15 increased the number of CD69+ NK cells, and neither IL-18 nor the neutralization of IL-10 modified the proportion of CD69+ NK cells or the expression of CD16 and CD11a molecules. Overall, these results indicate that the effects of IL-15 and IL-18 on NK cells from A-TB patients are not dependent on IL-10 modulation of the surface expression of activator/adhesion molecules.
Table 5.
Neutralization of interleukin (IL)-10 modified stimulatory molecules on CD3– CD56+ cells from patients with advanced tuberculosis (A-TB)
| MFI | |||
|---|---|---|---|
| PBMC incubated with | CD16 | CD11a | % CD69+ cells |
| – | 178 ± 27 | 190 ± 48 | 14 ± 5 |
| anti-IL-10 | 232 ± 30* | 315 ± 40* | 14 ± 6 |
| M.tb | 165 ± 36 | 162 ± 31 | 16 ± 5 |
| M.tb + anti-IL-10 | 206 ± 47* | 290 ± 46* | 30 ± 6* |
| M.tb + IL-15 | 219 ± 50 | 250 ± 35 | 56 ± 6 |
| M.tb + IL-15 + anti-IL-10 | 204 ± 31 | 310 ± 57 | 55 ± 6 |
| M.tb + IL-18 | 171 ± 36 | 250 ± 38 | 25 ± 5 |
| M.tb + IL-18 + anti-IL-10 | 166 ± 26 | 270 ± 46 | 28 ± 6 |
Peripheral blood mononuclear cells (PBMC) from four patients with advanced tuberculosis (A-TB) were incubated in complete medium (–) or Mycobacterium tuberculosis (M.tb) in the presence or absence of interleukin (IL)-15 or IL-18, with or without anti-IL-10, for 24 hr and cell surface expression of CD16, CD69 or CD11a on CD3– CD56+ cells was analysed by flow cytometry. Mean fluorescence intensity (MFI) for CD16 and CD11a molecules and the percentage of CD69+ cells are shown. Results are expressed as mean ± standard error of the mean (SEM). Statistical differences: PBMC + anti-IL10 versus PBMC without anti-IL-10:
P < 0·05.
M. tuberculosis down-regulates ICAM-1+ CD14+ cells
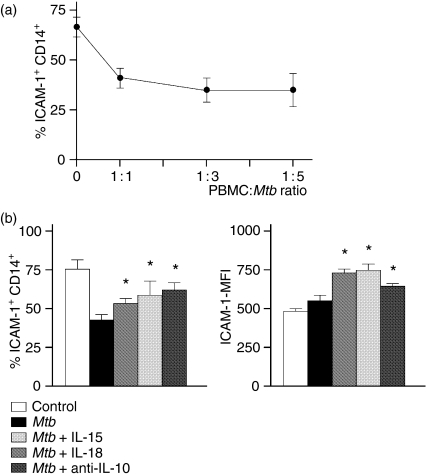
Considering that CD11a (LAF-1) is an adhesion molecule implicated as an early signal for NK cytotoxicity,4,19 and given that its expression is down-regulated in A-TB patients, we evaluated whether its ligand on monocytes, ICAM-1, may be modulated by M. tuberculosis and/or IL-15 or IL-18, or by neutralization of IL-10. Thus, PBMC from six TB patients (two M-TB and four A-TB) were incubated at different PBMC:M. tuberculosis ratios (1 : 1, 1 : 3 and 1 : 5) in the presence or absence of IL-15, IL-18 and anti-IL-10 for 18 hr, and ICAM-1 expression was determined on CD14+ cells. As shown in Fig. 1(a), the number of ICAM-1+ CD14+ cells decreased by between 25 and 40% at the 1 : 1 ratio and was not modified by higher bacterial concentrations. In addition, the number of ICAM-1+ CD14+ cells and ICAM-1 expression were increased by IL-15 or IL-18 or by neutralization of IL-10 in the presence of M. tuberculosis (Fig. 1b). Taken together, these results suggest that the decrease in the number of ICAM-1+ CD14+ cells produced by M. tuberculosis may modulate NK activation and lytic activity by affecting adhesion to NK cells.
Figure 1.
Mycobacterium tuberculosis (Mtb) regulated the number of intracellular adhesion molecule (ICAM)-1+ CD14+ cells and ICAM-1 expression. (a) Peripheral blood mononuclear cells (PBMC) from two patients with moderate tuberculosis (M-TB) and four patients with advanced tuberculosis (A-TB) were incubated for 18 hr at different PBMC to M. tuberculosis ratios and the percentage of ICAM-1+CD14+ cells was determined. Results are expressed as mean ± standard error of the mean (SEM). Statistical differences: PBMC +M. tuberculosis versus non-stimulated PBMC: *P < 0·01. (b) PBMC from six A-TB patients were incubated in complete medium (control) or M. tuberculosis in the presence or absence of interleukin (IL)-15, IL-18 or anti-IL-10 for 18 hr. The percentage of ICAM-1+ CD14+ cells and ICAM-1 expression [mean fluorescence intensity (MFI)] were then analysed by flow cytometry. Results are expressed as mean ± SEM. Statistical differences: PBMC + IL-15, IL-18 or anti-IL-10 versus PBMC without cytokines or anti-IL-10: *P < 0·05.
NK cell activity is down-regulated by monocytes in TB patients
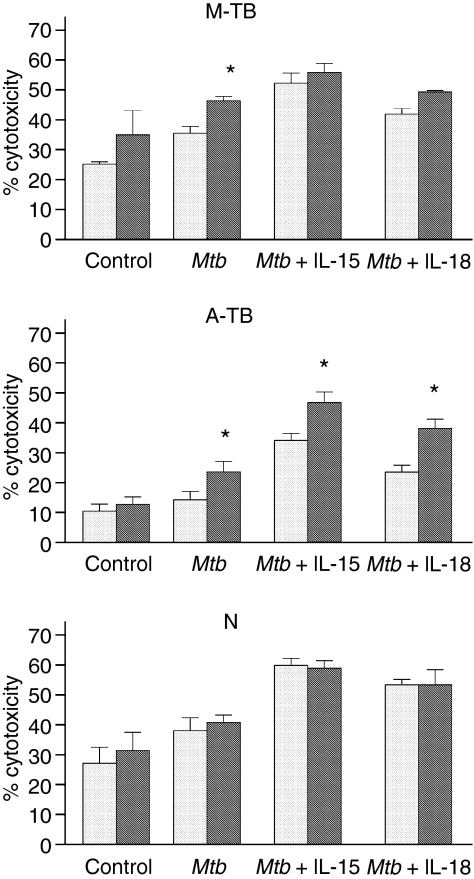
Given that monocytes play an important role in NK activation, not only through the release of cytokines but also by signalling through costimulatory molecules, we investigated whether monocytes could modulate M. tuberculosis-induced NK activation. Thus, PBMC depleted of monocytes were stimulated with M. tuberculosis in the presence or absence of IL-15, IL-18 and IL-10 for 18 hr and tested for NK activity and CD16, CD11a and CD69 expression. As shown in Fig. 2, an increase in M. tuberculosis-induced NK lytic activity in cells from TB patients was detected by depletion of monocytes, and furthermore, in the presence of IL-15 or IL-18, an up-regulation of NK function was observed in cells from A-TB patients. However, M. tuberculosis-induced NK function in N controls was not affected by monocyte depletion. Taken together, these results suggest a regulatory role of monocytes in NK activation in TB patients.
Figure 2.
Monocytes from patients with tuberculosis (TB) down-regulated natural killer (NK) activity. Peripheral blood mononuclear cells (PBMC) (grey bar) or monocyte-depleted mononuclear cells (black grey bar) from 12 patients with moderate TB (M-TB), 15 patients with advanced TB (A-TB) and 12 healthy individuals (N) were cultured in complete medium (control), Mycobacterium tuberculosis (Mtb) alone or M. tuberculosis plus interleukin (IL)-15 or IL-18 for 24 hr and tested for their lytic activity against K562 target cells. Results are expressed as percentage of cytotoxicity [mean ± standard error of the mean (SEM)]. Statistical differences: % cytotoxicity from monocyte-depleted cells versus % cytotoxicity from PBMC: *P < 0·05.
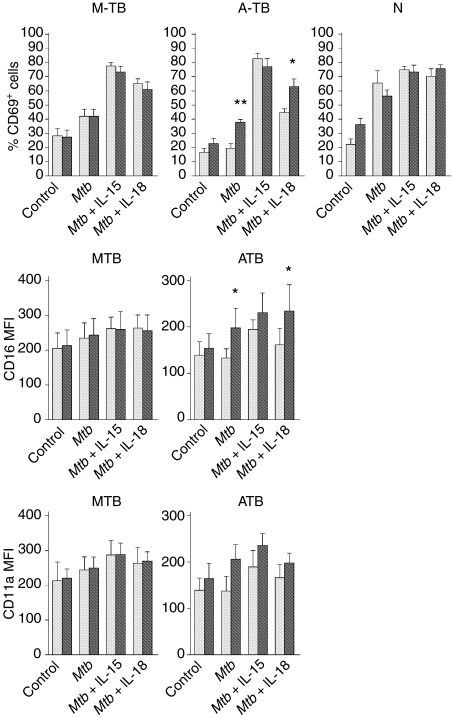
As shown in Fig. 3, monocyte depletion did not modify the proportion of CD69+ NK cells in control cells from TB patients or healthy individuals. However, M. tuberculosis stimulation in the absence of monocytes induced a decrease in the number of CD69+ NK cells from N controls and an increase in that from A-TB patients, and did not modify that from M-TB patients. Furthermore, while IL-15 had no effect on the number of CD69+ NK cells in TB and N controls in the absence of monocytes, the addition of IL-18 increased the percentage of CD69+ NK cells and enhanced the expression of the CD16 molecule in M. tuberculosis-stimulated cells from A-TB patients.
Figure 3.
Monocytes down-regulated activation molecules on natural killer (NK) cells from patients with advanced tuberculosis (A-TB). Peripheral blood mononuclear cells (PBMC) (grey bar) and monocyte-depleted mononuclear cells (grey black bar) from 10 patients with moderate tuberculosis (M-TB), 13 patients with advanced tuberculosis (A-TB) and eight healthy controls (N) were incubated in complete medium (control), Mycobacterium tuberculosis or M. tuberculosis plus interleukin (IL)-15 or IL-18 for 24 hr. The expression of CD16, CD69 and CD11a molecules on CD3– CD56+ cells was then determined by flow cytometry. Results are expressed as mean fluorescence intensity (MFI) for CD16 and CD11a molecules and percentage of CD69+ cells [mean ± standard error of the mean (SEM)]. Statistical differences: monocyte-depleted versus PBMC: *P < 0·05; **P < 0·01.
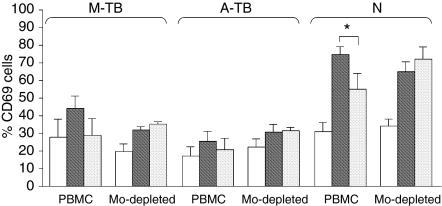
To assess whether IL-10 directly modulates the activation of NK cells, PBMC were depleted of CD14+ cells by magnetic methods and stimulated or not stimulated with M. tuberculosis in the presence or absence of IL-10 for 18 hr. Thereafter, the percentage of CD69+ cells (as a measurement of activation) and CD16 expression on CD3– CD56+ cells were determined by flow cytometry. As shown in Fig. 4, while in the presence of monocytes exogenous IL-10 down-regulated the activation of NK cells in M-TB patients and N controls, it had no effect on NK activation in the absence of monocytes. In addition, CD16 expression was not modified in NK cells by the depletion of CD14+ cells (data not shown). With regard to NK function, M. tuberculosis-induced NK lytic activity was increased by monocyte depletion in M-TB patients [PBMC (mean ± SEM): 33 ± 5; CD14-depleted: 49 ± 6, n = 3] and A-TB patients (PBMC: 10 ± 2; CD14-depleted: 24 ± 5, n = 3). Moreover, the addition of IL-10 to monocyte-depleted PBMC increased NK lytic activity in cells from M-TB patients (PBMC: 29 ± 4; CD14-depleted: 50 ± 6, n = 3) and did not modify that in cells from A-TB patients (PBMC: 10 ± 2; CD14-depleted: 16 ± 6, n = 3). Overall, these data indicate that IL-10 does not exert a direct effect on NK activation, suggesting an indirect effect through the down-regulation of costimulatory molecules on monocytes necessary for the activation of NK cells.
Figure 4.
Effect of interleukin (IL)-10 in the monocyte-depleted (Mo-depleted) CD69+ CD3– CD56+ population. Peripheral blood mononuclear cells (PBMC) or monocyte-depleted mononuclear cells from six patients with moderate tuberculosis (M-TB), six patients with advanced tuberculosis (A-TB) and five healthy individuals (N) were cultured in complete medium (white bar), Mycobacterium tuberculosis (grey black bar) or M. tuberculosis + IL-10 (grey bar) for 24 hr and the percentage of CD69+ on CD3– CD56+ cells was evaluated by flow cytometry. Results are expressed as mean ± standard error of the mean (SEM). Statistical differences: N-PBMC (Mtb vs Mtb + IL-10): *P < 0·05.
Numbers of M. tuberculosis-induced IFN-γ+ NK cells were increased by IL-15 and IL-18 in TB patients
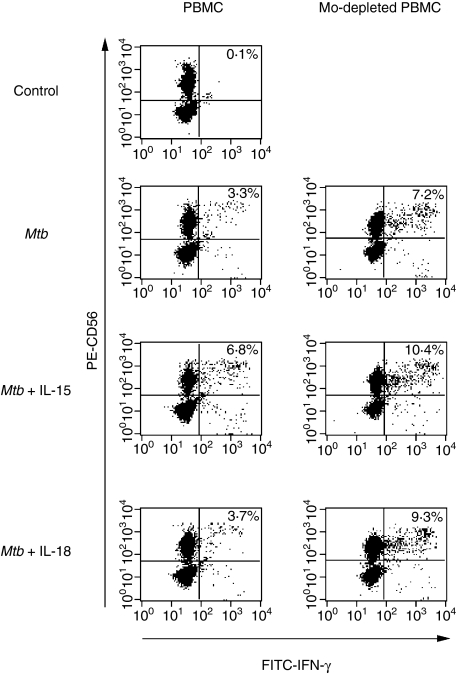
In the light of the known roles of IL-15 and IL-18 in the induction of IFN-γ production by NK cells, we tested whether the observed effect of these monokines on NK activity could be ascribed to IFN-γ production. For this purpose, PBMC from TB patients and N controls were stimulated or not stimulated with M. tuberculosis for 24 hr and IFN-γ production by CD3– CD56+ cells was analysed by flow cytometry. As shown in Table 6, no differences in IFN-γ production by non-stimulated (control) NK cells from TB patients and N controls were observed. However, upon M. tuberculosis stimulation, an increase in the proportion of IFN-γ+ CD3– CD56+ cells was detected for M-TB patients and N controls, while for A-TB patients no such increase was detected. Considering that IL-15 and IL-18 increased the number of CD69+ cells, up-regulated expression of the CD16 molecule and enhanced NK function in both PBMC and monocyte-depleted PBMC from A-TB patients, we assessed whether these findings could be ascribed to enhanced IFN-γ production. As shown in Fig. 5, the proportion of IFN-γ+ NK cells upon M. tuberculosis stimulation was increased by IL-15, even in the presence of monocytes, while IL-18 produced no such effect. However, IL-18 up-regulated the number of NK cells producing IFN-γ in M. tuberculosis-induced monocyte-depleted PBMC. Taken together, these data indicate that monocytes have a down-regulatory effect on IFN-γ-producing NK cells from A-TB patients.
Table 6.
Mycobacterium tuberculosis-induced interferon (IFN)-γ+ natural killer (NK) cells
| % IFN-γ+ NK cells | ||
|---|---|---|
| PBMC from | Control PBMC | M.tb-stimulated PBMC |
| M-TB (n = 5) | 1·0 ± 0·5 | 5·8 ± 0·8* |
| A-TB (n = 5) | 0·4 ± 0·3 | 2·5 ± 0·9 |
| N (n = 5) | 0·6 ± 0·3 | 7·5 ± 0·7* |
Peripheral blood mononuclear cells (PBMC) from patients with moderate tuberculosis (M-TB) and advanced tuberculosis (A-TB) and healthy controls (N) were incubated in complete medium (control) or with M. tuberculosis (M.tb) for 24 hr and then the percentage of IFN-γ+ cells on CD3– CD56+ cells was evaluated by flow cytometry. Results are expressed as mean ± standard error of the mean (SEM). Statistical differences: control versus M. tuberculosis-stimulated PBMC:
P < 0·05.
Figure 5.
Monocytes from patients with advanced tuberculosis (A-TB) modulated interferon (IFN)-γ+ production by CD3– CD56+ cells. Peripheral blood mononuclear cells (PBMC) and monocyte-depleted mononuclear cells (Mo-depleted) from three A-TB patients were incubated in complete medium (control), Mycobacterium tuberculosis (Mtb), or M. tuberculosis plus interleukin (IL)-15 or IL-18 for 24 hr and IFN-γ production by CD3– CD56+ cells was determined by flow cytometry. Results are expressed as the percentage of positive cells (data in the panels). A representative example is shown. FITC, fluorescein isothiocyanate.
Discussion
Although the role of NK cells in mycobacterial infections is unclear, in vitro studies have demonstrated that peripheral blood NK cells may contribute to protective immunity through the production of IFN-γ to maintain the frequency of M. tuberculosis-responsive CD8+ IFN-γ+ cells and expand their cytotoxic activity.22,23 In addition, NK cells mediate early killing of intracellular M. tuberculosis via granule-independent mechanisms.24
Our study demonstrating a higher proportion of NK cells in TB patients is in contrast with data previously reported.25,26 However, the decrease in lytic activity observed in NK cells from TB patients is in accordance with the results of studies employing either M. tuberculosis-infected macrophages or the K562 cell line as target cells.25,26 Moreover, a major reduction in NK lytic activity is observed in severe pulmonary involvement, indicating that the microenvironment might affect NK activation and/or function. In vitro studies have demonstrated that human NK cells can be activated either by mycobacterium (M. bovis) bacille Calmette–Guérin (BCG)27 or by lysed human monocytes infected with live M. bovis BCG28 or M. tuberculosis.29 The ability of NK cells to interact with target cells depends on the expression of cell surface receptors and adhesion molecules capable of binding to ligands on target cells. Herein, we have demonstrated that M. tuberculosis stimulates NK cells from healthy individuals, as shown by high expression of CD69 antigen and increases in natural cytotoxicity and IFN-γ production. However, M. tuberculosis stimulation of NK cells from TB patients showed impairment of their function, with the severity of pulmonary disease, with low numbers of CD69+ NK cells and an absence of M. tuberculosis-induced lysis and IFN-γ production, as was observed in A-TB patients. CD69 is a costimulatory molecule transiently expressed on activated lymphocytes,6 and the mechanism regulating its expression on NK cells is obscure. It has been demonstrated that CD69 is up-regulated after activation of CD16, LFA-1/CD11a, IL-2 or IFN-α receptors which signal through protein tyrosin kinase (PTK) phosphorylation and Vav/extracellular regulated kinase (Vav/ERK).7,30,31 Binding of the β2-integrin LFA-1 on NK cells to ICAM-1 on target cells initiates triggering signal transduction pathways3,4,32 and, furthermore, LFA-1 ligation costimulates the CD16 receptor for cytokine production.33 In addition to the role of CD16 in the killing of target cells by NK cells, its cross-linking initiates signalling events inducing granule exocytosis and cytokine production.18,34 Herein, we have demonstrated that the expression of CD11a and CD16, molecules implicated in the lysis of target cells, is down-regulated in cells from A-TB patients and, furthermore, that M. tuberculosis reduces the numbers of ICAM-1+ CD14+ cells in TB patients. However, in the presence of inflammatory cytokines or neutralization of IL-10, the number of ICAM-1+ CD14+ cells and ICAM-1 expression are enhanced, even in the presence of M. tuberculosis, along with an increase in NK activity. Given that conjugate formation between NK and target cells is a prerequisite for target cell lysis and is critically dependent on engagement of CD11a with its ligands on APCs, ICAM-1 regulation on monocytes from TB patients could be a mechanism used by M. tuberculosis to reduce NK activity.3,4,19,21,32 The finding that the expression of CD11a and CD16 molecules was associated in our study with the severity of the disease suggests that, in TB patients, the expression of costimulatory/activatory molecules and their biological functions could be conditioned by circulating cytokines and by those secreted upon M. tuberculosis stimulation by accessory cells.35,36 Therefore, the low expression of CD11a on NK cells, together with ICAM-1 down-regulation on monocytes by M. tuberculosis, may contribute to the impairment of NK activity in TB patients by diminishing the adhesiveness necessary for triggering signalling pathways downstream of CD11a/ICAM engagement. Although, to date, no receptor has been shown to be either necessary or sufficient in signalling for NK cell cytotoxicity, other receptors on NK cells, such as NKG2D and NKp46, and their ligands on target cells could be involved in the regulation of NK cell effector function, indicating a possible redundancy of activation receptors and activation pathways.2,37 In this context, natural cytotoxicity receptors may also be involved in the impairment of NK activity, as demonstrated for NKp46 in TB patients.25
It is well known that cytokines produced by accessory cells play an essential role in NK development and activation. IL-15 promotes in vitro NK cell development, mediates LFA-1 expression,38 and enhances the lytic activity and proliferation of resting NK cells as well as the production of cytokines and chemokines.13 While IL-18 increases the lytic activity of NK cells by up-regulating perforin-dependent cytotoxic pathways and Fas ligand (FasL) expression,39,40 the role of IL-10 in NK activation is not clear.41,42 Our results showed that, in effect, IL-15 enhanced M. tuberculosis-induced NK cytotoxicity in healthy individuals, inducing CD69 expression on NK cells just as M. tuberculosis does. However, IL-15 also up-regulated the expression of CD16 and CD11a on M. tuberculosis-stimulated NK cells from A-TB patients, in addition to increasing NK cytotoxicity in both groups of TB patients in association with a high number of CD69+ NK cells. In contrast to IL-15, IL-18 did not modify NK cytotoxicity in A-TB patients but slightly up-regulated the expression of CD69 and CD11a molecules. IL-10 neutralization in PBMC from TB patients increased M. tuberculosis-induced NK cytotoxicity and up-regulated CD16 and CD11a expression, suggesting that endogenous IL-10 could inhibit NK activation and function through the down-regulation of ICAM-1 on monocytes. Therefore, the increase in costimulatory molecules could enhance the efficiency of NK lysis by increasing the interaction with target cells and triggering granule exocytosis.
IL-10 can boost IFN-γ production by isolated NK cells,41,43 but in the presence of APCs it inhibits IFN-γ production.20,42 In this context, our data for monocyte-depleted populations demonstrated that M. tuberculosis directly triggered the activation of NK cells from both TB patients and healthy controls, as previously reported with pathogenic and non-pathogenic bacteria.27,44 Moreover, in the absence of monocytes, M. tuberculosis-induced NK cell activity was increased in TB patients while in N controls it was not. In addition, CD69 and CD16 expression on M. tuberculosis-stimulated NK cells was also increased in cells from A-TB patients, and IL-18 up-regulated their expression while IL-15 did not, demonstrating the different roles of these two cytokines in NK function.38–40 Furthermore, in TB patients, the modulatory effect of monocytes was also observed in the production of IFN-γ by M. tuberculosis-stimulated NK cells, where the lowest levels detected in A-TB patients were increased by the depletion of monocytes. Although IFN-γ is not required for NK cytotoxicity and does not mediate the killing of intracellular M. tuberculosis,24,38 it may contribute to switching the immune response towards type 1 by enhancing the production of IL-12 and IL-18 in response to M. tuberculosis.47,48 Taken together, these results confirm a regulatory role of monocytes in NK cell activation and function in TB patients.
In conclusion, we have demonstrated that monocytes from TB patients regulate NK function involving IL-10, which acts through an indirect mechanism leading to down-regulation of costimulatory/adhesion molecules and/or the IFN-γ production necessary for the activation of NK cells. Therefore, our findings demonstrate an underlying role of monocytes in NK function in TB patients.
Acknowledgments
We thank the medical staff of the División Tisioneumonología, Hospital F. J. Muñiz for their substantial help in providing clinical samples from patients with tuberculosis. This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, 05-14060) and CONICET.
References
- 1.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 2.Moretta L, Bottino C, Pende D, Mingari MC, Blassoni R, Moretta A. Human natural killer cells: their origin, receptors and function. Eur J Immunol. 2002;32:1205–11. doi: 10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 3.Perez OD, Mitcheli D, Jager G, Nolan G. LFA-1 signaling through p44/42 is coupled to perforin degranulation in CD56+CD8+ natural killer cells. Blood. 2004;104:1083–93. doi: 10.1182/blood-2003-08-2652. [DOI] [PubMed] [Google Scholar]
- 4.Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653–9. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler SF, Ramsdell F, Hjerrild KA, et al. Molecular characterization of the early activation CD69: a type II membrane glycoprotein related to a family of natural killer cell activation antigens. Eur J Immunol. 1993;23:1643–8. doi: 10.1002/eji.1830230737. [DOI] [PubMed] [Google Scholar]
- 6.Sancho D, Gómez M, Sánchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–40. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Borrego F, Pena J, Solana R. Regulation of CD69 expression on natural killer cells: differential involvement of protein kinase C and protein tyrosine kinases. Eur J Immunol. 1993;23:1039–43. doi: 10.1002/eji.1830230509. [DOI] [PubMed] [Google Scholar]
- 8.Fehniger TA, Shah MH, Turner MJ, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–20. [PubMed] [Google Scholar]
- 9.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between Natural Killer cells and dendritic cells. J Exp Med. 2002;195:327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehninger TA, Caligiuri M. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 12.Chan SH, Perussia B, Gupta JW, et al. Induction of interferon γ production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–79. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-γ production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:159–64. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 15.Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, Grabstein K, Caligiuri MA. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-γ by natural killer cells in vitro. J Clin Invest. 1995;96:2578–82. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Invest. 1968;97:77–89. [PubMed] [Google Scholar]
- 17.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells. a unique innate immunoregulatory role for the CD56 (bright) subset. Blood. 2001;97:3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 18.Milella M, Gismondi A, Roncaioli P, Bisogno L, Palmieri G, Frati L, Cifone MG, Santoni A. CD16 cross-linking induces both secretory and extracellular signal-regulated kinase (ERK)-dependent cytosolic phospholipase A2 (PLA2) activity in human natural killer cells. involvement of ERK, but not PLA2, in CD16-triggered granule exocytosis. J Immunol. 1997;158:3148–54. [PubMed] [Google Scholar]
- 19.Helander TS. Adhesion in NK cell function. Curr Top Microbiol Immunol. 1998;230:89–98. doi: 10.1007/978-3-642-46859-9_7. [DOI] [PubMed] [Google Scholar]
- 20.Goodier MR, Londei M. Lypopolysaccharide stimulates the proliferation of human CD56+CD3– NK cells: a regulatory role of monocytes and IL-10. J Immunol. 2000;165:139–47. doi: 10.4049/jimmunol.165.1.139. [DOI] [PubMed] [Google Scholar]
- 21.de la Barrera S, Aleman M, Musella R, Schierloh P, Pasquinelli V, Garcia V, Abbate E, del Sasiain MC. IL-10 down-regulates costimulatory molecules on Mycobacterium tuberculosis-pulsed macrophages and impairs the lytic activity of CD4 and CD8 CTL in tuberculosis patients. Clin Exp Immunol. 2004;138:128–38. doi: 10.1111/j.1365-2249.2004.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 23.Vankayalapati R, Klucar P, Wizel B, Weiz SE, Samtem B, Safi H, Shams H, Barnes PF. NK cells regulate CD8+ T cell effector function in response to an intracellular pathogen. J Immunol. 2004;172:130–7. doi: 10.4049/jimmunol.172.1.130. [DOI] [PubMed] [Google Scholar]
- 24.Brill KJ, Li Q, Larkin R, Canaday DH, Kaplan DR, Boom WH, Silver RF. Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms. Infect Immun. 2001;69:1755–65. doi: 10.1128/IAI.69.3.1755-1765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vankayalapati R, Wizel B, Weis SE, et al. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168:3451–7. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- 26.Nirmala R, Narayanan PR, Mathew R, Maran M, Deivanayagam CN. Reduced NK activity in pulmonary tuberculosis patients with/without HIV infection. Identifying the defective stage and studying the effect of interleukins on NK activity. Tuberculosis. 2001;81:343–52. doi: 10.1054/tube.2001.0309. [DOI] [PubMed] [Google Scholar]
- 27.Esin S, Batoni G, Pardini M, et al. Functional characterization of human natural killer cells responding to Mycobacterium bovis bacille Calmette-Guerin. Immunology. 2004;112:143–52. doi: 10.1111/j.1365-2567.2004.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molloy A, Meyn PA, Smith KD, Kaplan G. Recognition and destruction of Bacillus Calmette-Guerin-infected human monocytes. J Exp Med. 1993;177:1691–8. doi: 10.1084/jem.177.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denis M. Interleukin-12 (IL-12) augments cytolytic activity of natural killer cells toward Mycobacterium tuberculosis-infected human monocytes. Cell Immunol. 1994;156:529–36. doi: 10.1006/cimm.1994.1196. [DOI] [PubMed] [Google Scholar]
- 30.Villaba M, Hernandez J, Deckert M, Tanaka Y, Altman A. Vav modulation of the Ras/MEK/ERK signaling pathway plays a role in NFAT activation and CD69 up-regulation. Eur J Immunol. 2000;30:1587–96. doi: 10.1002/1521-4141(200006)30:6<1587::AID-IMMU1587>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 31.Sulica A, Metes D, Gherman M, Whiteside TL, Herberman RB. Divergent effects of Fc gamma RIIIA ligands on the functional activities of human natural killer cells in vitro. Eur J Immunol. 1996;26:1199–203. doi: 10.1002/eji.1830260602. [DOI] [PubMed] [Google Scholar]
- 32.Melero I, Balboa MA, Alonso JL, Yague E, Pivel JP, Sanchez-Madrid F, Lopez-Botet M. Signaling through the LFA-1 leukocyte integrin actively regulates intercellular adhesion and tumor necrosis factor-alpha production in natural killer cells. Eur J Immunol. 1993;23:1859–65. doi: 10.1002/eji.1830230819. [DOI] [PubMed] [Google Scholar]
- 33.Riteau B, Barber DF, Long EO. Vav1 phosphorylation is induced by beta2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med. 2003;198:469–74. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE, Strominger JL. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc Natl Acad Sci USA. 1999;96:5640–4. doi: 10.1073/pnas.96.10.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lozza MJ, Perussia B. The IL-12 signature: NK cell terminal CD56+ high stage and effector functions. J Immunol. 2004;172:88–96. doi: 10.4049/jimmunol.172.1.88. [DOI] [PubMed] [Google Scholar]
- 36.Dlugovizky DA, Torrez-Morales A, Rateni L, Farroni MA, Largacha O, Molteni O, Bottasso O. Circulating profile of Th1 and Th2 cytokines in tuberculosis patients with different degrees of pulmonary involvement. FEMS Immunol Med Microbiol. 1997;18:203–7. doi: 10.1111/j.1574-695X.1997.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 37.Smyth MK, Cretney E, Kelly JM, et al. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42:501–10. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Barao I, Hudig D, Ascensao JL. IL-15 mediated induction of LFA-1 is a late step required for cytotoxic differentiation of human NK cells from CD34+Lin– bone marrow cells. J Immunol. 2003;171:683–90. doi: 10.4049/jimmunol.171.2.683. [DOI] [PubMed] [Google Scholar]
- 39.Hyodo Y, Matsui K, Hayashi N, et al. IL-18 upregulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding constitutively expressed IL-18 receptor. J Immunol. 1999;162:1662–8. [PubMed] [Google Scholar]
- 40.Takeda K, Tsutsui H, Yoshimoto T, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–90. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 41.Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFNγ when combined with IL-18. Eur J Immunol. 1999;29:2658–65. doi: 10.1002/(SICI)1521-4141(199909)29:09<2658::AID-IMMU2658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 42.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin-10 (IL-10) inhibits human lymphocyte interferon-γ production by suppressing natural killer stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibata Y, Foster IA, Kurimoto M, et al. Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-γ inducing factors but enhances NK cell production of IFN-γ. J Immunol. 1998;161:4283–8. [PubMed] [Google Scholar]
- 44.Scotts MJ, Hoth JJ, Stagner MK, Gardner SA, Peyton JC, Cheadle WG. CD40–CD154 interactions between macrophages and natural killer cells during sepsis are critical for macrophage activation and are not interferon gamma dependent. Clin Exp Immunol. 2004;137:469–77. doi: 10.1111/j.1365-2249.2004.02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsutsui N, Nakanishi K, Matsui K, Higashino H, Okamura Y, Miyazawa Kaneda K. Interferon-γ-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J Immunol. 1996;157:3967–73. [PubMed] [Google Scholar]
- 46.Haller D, Blum S, Bode C, Hammes WO, Schiffrin EJ. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infect Immun. 2000;68:752–9. doi: 10.1128/iai.68.2.752-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flesh IEA, Hess JH, Huang M, Auget J, Rothe H, Bluethmann H, Kaufmann SHE. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon γ and tumor necrosis factor α. J Exp Med. 1995;181:1615–21. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vankayalapati R, Wizel B, Lackey DL, Zhang Y, Coffe KA, Griffith DE, Barnes PF. T cells enhance production of IL-18 by monocytes in response to an intracellular pathogen. J Immunol. 2001;166:6749–53. doi: 10.4049/jimmunol.166.11.6749. [DOI] [PubMed] [Google Scholar]