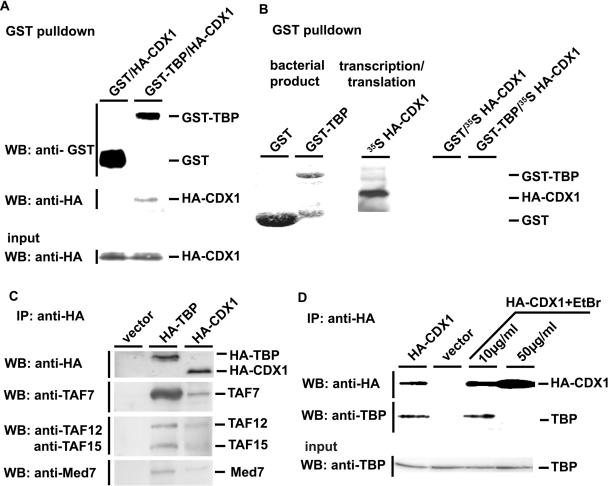
Figure 2.
Characterization of the CDX1/TBP interaction. (A) Interaction of bacterially-produced GST-TBP and cellular HA-CDX1. GST or GST-TBP produced in bacteria was bound to glutathione–Sepharose-4B beads [see also the first two lanes in (B)] and mixed with HCT116 cells extracts containing HA-CDX1. After GST-pulldown, the presence of GST and GST-TBP was revealed using anti-GST antibody, and the presence of HA-CDX1 was detected using anti-HA antibody. The presence of HA-CDX1 in the cell extracts prior to GST-pulldown was control by western blot with anti-HA antibody (Input). (B) Lack of interaction between bacterially-produced GST-TBP and in vitro translated HA-CDX1. GST (first lane, Coomasie blue staining) and GST-TBP (second lane, Coomasie blue staining) were produced in bacteria and bound to glutathione–Sepharose-4B beads. 35S-Methionine-labeled HA-CDX1 was synthesized by in vitro transcription/translation (third lane, autoradiography). By mixing GST and HA-CDX1 (fourth lane) or GST-TBP and HA-CDX1 (fifth lane), no interaction was detected after GST-pulldown and autoradiography. (C) CDX1 interaction with components of the TFIID and Med complexes. HCT116 cells were transfected with the control empty vector or with the plasmids encoding HA-TBP or HA-CDX1. Proteins immunoprecipitated with anti-HA antibody were detected by western blots using the antibodies raised against TAF7, TAF12, TAF15 and Med7. D. Effect of ethidium bromide (EtBr) on the CDX1/TBP interaction. HCT116 were transfected with the plasmid encoding HA-CDX1 and the proteins were immunoprecipitated with anti-HA antibody in the presence of increasing amount of ethidium bromide. Western blot with anti-HA antibody was used to control the step of immunoprecipitation and anti-TBP was used to detect the co-immunoprecipitated TBP. The presence of TBP in every cell extract prior to co-immunoprecipitation was controlled using anti-TBP antibody (Input).