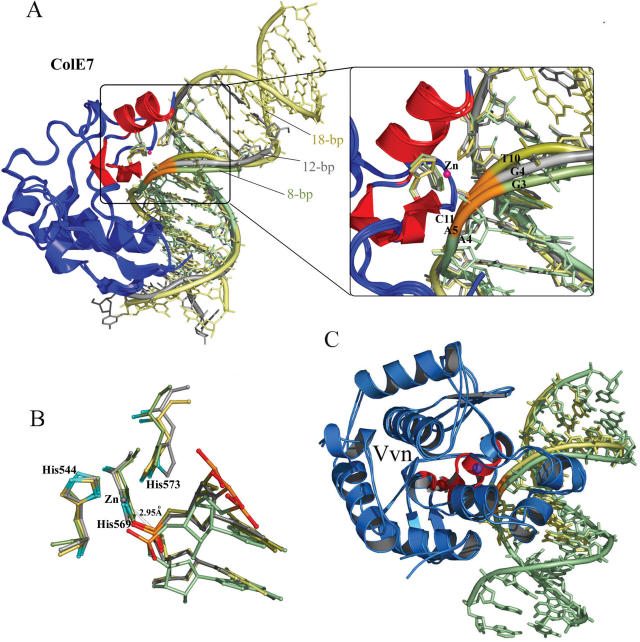
Figure 5.
Superposition of three N-ColE7–DNA complexes and two Vvn–DNA complexes. (A) The crystal structures of the three N-ColE7–DNA complexes, the preferred complex (18 bp: 2IVH) and the non-preferred complexes (8 bp: 1PT3 and 12 bp: 1ZNS), were superimposed by least-square-fitting of the Cα-atoms of N-ColE7 molecules. The three duplex DNA were aligned after fitting with the metal finger motif (displayed in red) bound at the minor groove, inducing DNA to bend slightly away from the enzyme. The DNA backbones are displayed in yellow for the 18 bp DNA, gray for 12 bp DNA and green for 8 bp DNA, but the backbone ribbons of the scissile phosphates are all displayed in orange. A detailed view of the phosphate backbones is shown in the right panel. The DNA backbone is distorted the most in the preferred complex with a thymine base (T10) located at the 3′-O side of the scissile phosphate as compared to the backbones in the non-preferred complexes, which contain a guanine base before the scissile phosphates (G4 in the 12 bp complex and G3 in the 8 bp complex). (B) Superposition of the active sites in the three N-ColE7–DNA complexes shows that the scissile phosphate is located closer to the endonuclease active site in the preferred complex (18 bp) with a distance of 2.95 Å between the zinc ion and phosphorus atom. (C) The crystal structures of Vvn–DNA complexes, 8 bp (PDB: 1OUP) and 16 bp (this study, PDB: 2IVK) complexes, were superimposed by a least-square-fit of the Cα atoms in Vvn. The DNA backbones fitted well and were not distorted, consistent with Vvn's low sequence-dependent endonuclease activity. Vvn are displayed in blue with metal finger motifs in red. The 16 bp DNA is displayed in green and 8 bp DNA is displayed in yellow.