Table 1.
Glycosidic Bond Forming Reactions
| Activator
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (BSP) | 2 | 3 | |||||||||
| Acceptor | Donor | Product | Yield (%) | α:β ratio | Yield (%) | α:β ratio | Yield (%) | α:β ratio | |||
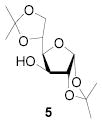
|
|
|
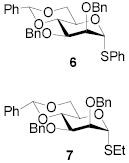
77 | 1:>9 | 90 | 1:>9 | 87 | 1:>9 | |||
| - | - | 91 | 1:>9 | 89 | 1:>9 | ||||||
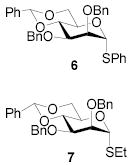
|
|
|
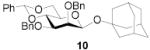
88 | 1:>9 | 88 | 1:>9 | - | - | |||
| - | - | 94 | 1:>9 | 99 | 1:>9 | ||||||
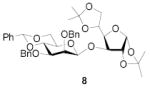
|
|
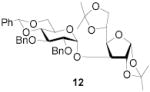
|
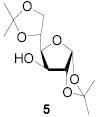
74 | >9:1 | 89 | 8.3:1 | 82 | 7.3:1 | |||
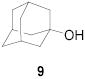
|
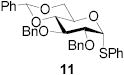
|
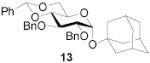
|
72 | >9:1 | 91a | 7.0:1 | 86a | 6.2:1 | |||
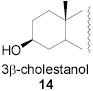
|
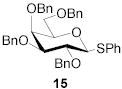
|
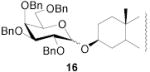
|
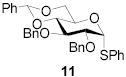
78 | 1:1 | 87 | 1:3.0 | 87 | 1:3.9 | |||
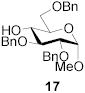
|
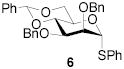
|
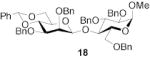
|
- | - | 83 | 1:>9 | - | - | |||
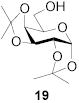
|
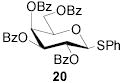
|
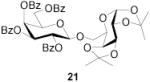
|
78b | 1:>9 | 85 | 1:>9 | 80 | 1:>9 | |||
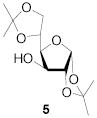
|
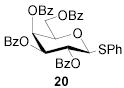
|
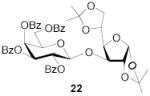
|
70b | 1:>9 | 76b | 1:>9 | 73b | 1:>9 | |||
Reaction was performed under dilute conditions (0.01 M donor dichloromethane) as this led to enhanced α:β ratio;
Reaction was conducted at −40 °C in order to achieve rapid activation of this disarmed donor.
