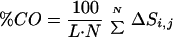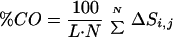Comparison of calculated and experimentally observed folding rates at zero denaturant concentration in the double sequence approximation. The observed rates are taken from the compilation by Jackson (
3). The correlation coefficient in the single, double, and triple sequence approximations are 0.83, 0.85, and 0.87, respectively. In the first two, the parameters of the model were adjusted to maximize the agreement by using a least squares criterion. The rates in the triple sequence approximation were calculated with the values of the two Δ
sk’s and
D from the double sequence approximation, and a new set of ɛ’s in order to reproduce the experimental equilibrium constants. A plot of the experimental log
kf versus log
K gives a correlation coefficient of 0.25, showing no significant correlation between folding rate and thermodynamic stability in this set of proteins. The inset shows a plot of the experimental rates versus the percent contact order (%CO) calculated using 0.4 nm as the cut-off-distance for the 18 two-state proteins of our study. The percent contact order defined by Plaxco
et al. (
19) is
where
N is the total number of contacts, Δ
Si,j is the sequence separation in residues between contacting residues
i and
j, and
L is the total number of residues in the protein. The correlation coefficient is 0.64. The folding rate for the β-hairpin extrapolated from the least-squares line is almost 10
8× smaller than the experimental value. Without the length normalization (
L), however, the extrapolated rate is only 30× slower.